Immunological consequences of kidney cell death - PubMed (original) (raw)
Review
Immunological consequences of kidney cell death
Maysa Sarhan et al. Cell Death Dis. 2018.
Abstract
Death of renal cells is central to the pathophysiology of acute tubular necrosis, autoimmunity, necrotizing glomerulonephritis, cystic kidney disease, urosepsis, delayed graft function and transplant rejection. By means of regulated necrosis, immunogenic damage-associated molecular patterns (DAMPs) and highly reactive organelles such as lysosomes, peroxisomes and mitochondria are released from the dying cells, thereby causing an overwhelming immunologic response. The rupture of the plasma membrane exhibits the "point of no return" for the immunogenicity of regulated cell death, explaining why apoptosis, a highly organized cell death subroutine with long-lasting plasma membrane integrity, elicits hardly any immune response. Ferroptosis, an iron-dependent necrotic type cell death, results in the release of DAMPs and large amounts of lipid peroxides. In contrast, anti-inflammatory cytokines are actively released from cells that die by necroptosis, limiting the DAMP-induced immune response to a surrounding microenvironment, whereas at the same time, inflammasome-associated caspases drive maturation of intracellularly expressed interleukin-1β (IL-1β). In a distinct setting, additionally interleukin-18 (IL-18) is expressed during pyroptosis, initiated by gasdermin-mediated plasma membrane rupture. As all of these pathways are druggable, we provide an overview of regulated necrosis in kidney diseases with a focus on immunogenicity and potential therapeutic interventions.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures
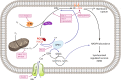
Fig. 1. The signalling pathway of ferroptosis
Peroxidation of membrane lipids, predominantly phosphatidylinositole and phosphotidylethanolamine, represents the point of no return during ferroptosis that results in loss of NADPH abundance and synchronized regulated necrosis (SRN) in sensitive organs, such as the renal tubular compartment or the myocardium. Lipoxygenases (ALOX) mediate lipid peroxidation, predominantly and specifically of PIP2 and phosphatidylethanolamine (PE). The constitutively active function of glutathione peroxidase 4 (GPX4), a selenoenzyme that requires glutathione (GSH) to function, prevents lipid peroxidation. Ferroptosis may be triggered by inhibition of system Xc-minus, a cys/glu-antiporter in the plasma membrane by a lethal compound referred to as erastin. Inhibition of system Xc-minus functionally inhibits the activation of the GSH-synthase (GSSG), resulting in GSH depletion, dysfunction of GPX4 and ferroptosis. RSL3 induces ferroptosis directly by inhibition of GPX4, and certain nanoparticles are capable of inducing ALOX activation in vitro. Before lipid peroxidation occurs, it may be prevented by the ferrostatins liproxstatin, ferrostatin-1 (Fer-1), necrostatin-1 (Nec-1), and the novel compounds 16–86, XJB-5-131 and JP4-039. The latter carries intrinsic anti-necroptotic activity beyond its function as a ferrostatin. Ferroptosis accounts for the majority of tubular cell loss during acute kidney injury because of its mechanism of SRN that shuts down an entire functional unit, but ferroptosis may be triggered by any cell death that occurs in cells of the functional syncytium because of the preterminal loss of NADPH that occurs in all cell death pathways
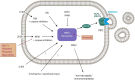
Fig. 2. Necroptosis
Phosphorylated mixed linage kinase domain like (pMLKL) is the only known mediator of necroptosis and its detection defines the activation of this pathway. Potentially, several kinases may phosphorylate MLKL, but only receptor-interacting protein kinase 3 (RIPK3) has been described while this review was written. MLKL carries several phosphorylation sites, and RIPK3 phosphorylates the so-called “activation loop”. However, complete function of pMLKL requires the depohsphorylation at the hinge region that unleashes the deadly activity of a four-helical bundle (4-HB), which binds PIP2 in the plasma membrane and tends to oligomerize with other pMLKL molecules. Downstream of pMLKL, sensitivity of cells to undergo necroptosis is controlled by proteins that control membrane blebbing and microvesical formation. Unlike previously suggested, pMLKL does not directly form pores in the plasma membrane of cells. Full activation of RIPK3 requires the assembly of the necrosome, a higher order structure that consists of oligomerized RIPK3 molecules that are stabilized by HSP90 and CDC-37, two chaperones the loss of which results in defective necroptosis. Several triggers result in the formation of the necrosome. Death receptor (DR), for example, TNFR1-stimulation in the presence of a caspase inhibitor or a dysfunctional caspase-8 represents the most prominent and best investigated stimulus that requires RIPK1 kinase activity for necrosome formation. RIPK1, as RIPK3, contains a rip homotypic-interacting motif (RHIM)-domain that intercalates with the RHIM of RIPK3 and prevents necrosome assembly. Inhibitors of RIPK1 kinase activity, such as Nec-1s and ponatinib, maintain the inactive state of RIPK3 potentially by keeping RHIM–RHIM interactions intact. Necrosome assembly has been repeatedly reported downstream of Toll-like receptors that bind to the intracellular adapter protein TRIF, which also contains a RHIM domain and activation of this pathway results in robust RIPK1-independent necrosome formation. In vivo, reperfusion following ischemic injury severely triggers necroptosis, and several other models have been described, such as injection of recombinant human TNFα into mice. In addition, protein kinase R (PKR) and DAI, a protein that is capable of but functionally not limited to viral DNA sensing activation as triggered by interferons, can activate RIPK1-mediated necrosome formation, but the relative contribution of these two factors remains unclear. Certainly, DAI robustly triggeres necrosome formation via its RHIM domain, possibly via nuclear signalling. However, importantly, necroptosis contributes to acute kidney injury in some models, such as ischemia, but inhibition of necroptosis does not affect other models, such as foliac acid-induced AKI
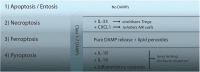
Fig. 3. Specific DAMP release defines an inflammatory hierarchy of cell death pathways
For a single cell, the mode of cell death does not matter, but it does matter for the environment. The apoptotic program contains several features that prevent its immunogenicity, including the persistence of plasma membrane integrity that prevents the release of DAMPs. We therefore consider apoptosis not at all immunogenic. In contrast to apoptosis, all subroutines of regulated necrosis result in the release of DAMPs because of plasma membrane rupture, and therefore all pathways of regulated necrosis (RN) represent highly immunogenic stimuli. Within the family of RN pathways, modulation of the immune response beyond DAMP release is common and results in a hierarchy of immunogenicity of RN pathways. During necroptosis, IL-33 and CXCL-1 are actively produced in an energy dependent manner, resulting in ST2-mediated stabilization of regulatory T cells and the Mincle-mediated inhibition of innate immunity (e.g., NK cells), respectively. Therefore, necroptosis, apart from releasing DAMPs, inhibits the immune response. During ferroptosis, lipid peroxides may predominate and add an immunogenic component to the DAMPs. However, the most immunogenic RN pathway appears to be pyroptosis due to its active maturation of long-lasting cytokines IL-1β and IL-18 alongside with the release of inflammatory caspases
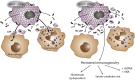
Fig. 4. Failure to remove necrotic debris results in autoimmunity
Macrophages and granulocytes remove necrotic debris by a process of LC3-associated phagocytosis (LAP), also referred to as noncanonical autophagy. Failure to remove necrotic debris results in persistence of DAMP-mediated immunogenicity. Over the time of months to 1 year, mice deficient in LAP (rubicon-ko mice) develop a lupus-like disease including immunoglobulin deposition in glomerula, increased serum levels of creatinine and urea, and development of autoantibodies against double-stranded DNA and antinuclear antibodies (ANAs)
Fig. 5. A novel hypothesis for the development of antibody-mediated rejection (ABMR)
Upon the process of transplantation of damaged (e.g., marginal) organs, naive recipient B cells encounter massive necrosis and DAMPs during the very first passage through the graft and are being potently primed. The proliferative B-cell response and the subsequent differentiation to plasma cells is prevented by the standard immunosuppression at this early time point. However, upon tapering of the immunosuppression during the first year after virtually successful transplantation, primed memory B cells progressively proliferate and terminally differentiate into plasma cells, producing donor-specific antibodies and mediating ABMR. Clinically, at this stage, plasma exchange may temporarily prevent ABMR progression, but as soon as plasma exchange stops, ABMR will slowly reactivate. Our model suggests that early interference with regulated necrosis unlike HLA- or even blood group-matching possesses the capacity to prevent memory B-cell priming and ABMR. Addition of necrosis blocking agents, for example, small molecules in the machine perfusate, are predicted to prevent ABMR
Similar articles
- Origin and Consequences of Necroinflammation.
Sarhan M, Land WG, Tonnus W, Hugo CP, Linkermann A. Sarhan M, et al. Physiol Rev. 2018 Apr 1;98(2):727-780. doi: 10.1152/physrev.00041.2016. Physiol Rev. 2018. PMID: 29465288 Review. - Cell Death Pathways Drive Necroinflammation during Acute Kidney Injury.
von Mässenhausen A, Tonnus W, Linkermann A. von Mässenhausen A, et al. Nephron. 2018;140(2):144-147. doi: 10.1159/000490807. Epub 2018 Jun 29. Nephron. 2018. PMID: 29961062 Review. - An Overview of Pathways of Regulated Necrosis in Acute Kidney Injury.
Kers J, Leemans JC, Linkermann A. Kers J, et al. Semin Nephrol. 2016 May;36(3):139-52. doi: 10.1016/j.semnephrol.2016.03.002. Semin Nephrol. 2016. PMID: 27339380 Review. - The NLRP3 inflammasome in kidney disease and autoimmunity.
Hutton HL, Ooi JD, Holdsworth SR, Kitching AR. Hutton HL, et al. Nephrology (Carlton). 2016 Sep;21(9):736-44. doi: 10.1111/nep.12785. Nephrology (Carlton). 2016. PMID: 27011059 Review. - Targeting of regulated necrosis in kidney disease.
Martin-Sanchez D, Poveda J, Fontecha-Barriuso M, Ruiz-Andres O, Sanchez-Niño MD, Ruiz-Ortega M, Ortiz A, Sanz AB. Martin-Sanchez D, et al. Nefrologia (Engl Ed). 2018 Mar-Apr;38(2):125-135. doi: 10.1016/j.nefro.2017.04.004. Epub 2017 Jun 21. Nefrologia (Engl Ed). 2018. PMID: 28647049 English, Spanish.
Cited by
- The significance of ferroptosis in renal diseases and its therapeutic potential.
Jiang M, Wu S, Xie K, Zhou G, Zhou W, Bao P. Jiang M, et al. Heliyon. 2024 Aug 6;10(16):e35882. doi: 10.1016/j.heliyon.2024.e35882. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39220983 Free PMC article. Review. - Development and evaluation of a chronic kidney disease risk prediction model using random forest.
Mendapara K. Mendapara K. Front Genet. 2024 Jun 27;15:1409755. doi: 10.3389/fgene.2024.1409755. eCollection 2024. Front Genet. 2024. PMID: 38993480 Free PMC article. - Cellular and molecular mechanisms of cell damage and cell death in ischemia-reperfusion injury in organ transplantation.
Dugbartey GJ. Dugbartey GJ. Mol Biol Rep. 2024 Mar 29;51(1):473. doi: 10.1007/s11033-024-09261-7. Mol Biol Rep. 2024. PMID: 38553658 Free PMC article. Review. - Regulated necrosis role in inflammation and repair in acute kidney injury.
Guerrero-Mauvecin J, Villar-Gómez N, Rayego-Mateos S, Ramos AM, Ruiz-Ortega M, Ortiz A, Sanz AB. Guerrero-Mauvecin J, et al. Front Immunol. 2023 Nov 24;14:1324996. doi: 10.3389/fimmu.2023.1324996. eCollection 2023. Front Immunol. 2023. PMID: 38077379 Free PMC article. Review. - TLR2 mediates renal apoptosis in neonatal mice subjected experimentally to obstructive nephropathy.
Wyczanska M, Rohling J, Keller U, Benz MR, Kirschning C, Lange-Sperandio B. Wyczanska M, et al. PLoS One. 2023 Nov 28;18(11):e0294142. doi: 10.1371/journal.pone.0294142. eCollection 2023. PLoS One. 2023. PMID: 38015955 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous