Epigenetic control of CD8+ T cell differentiation - PubMed (original) (raw)
Review
Epigenetic control of CD8+ T cell differentiation
Amanda N Henning et al. Nat Rev Immunol. 2018 May.
Abstract
Upon stimulation, small numbers of naive CD8+ T cells proliferate and differentiate into a variety of memory and effector cell types. CD8+ T cells can persist for years and kill tumour cells and virally infected cells. The functional and phenotypic changes that occur during CD8+ T cell differentiation are well characterized, but the epigenetic states that underlie these changes are incompletely understood. Here, we review the epigenetic processes that direct CD8+ T cell differentiation and function. We focus on epigenetic modification of DNA and associated histones at genes and their regulatory elements. We also describe structural changes in chromatin organization that affect gene expression. Finally, we examine the translational potential of epigenetic interventions to improve CD8+ T cell function in individuals with chronic infections and cancer.
Conflict of interest statement
Competing interests statement
The authors declare no competing interests.
Figures
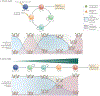
Figure 1 |. Different CD8 + T cell differentiation models result in unique transcriptional and epigenetic patterns over time.
a | In the On–Off–On, or circular, model of CD8+ T cell differentiation, effector T (TEFF) cells represent biological intermediaries that either undergo apoptosis or differentiate into memory T cell subsets following antigen withdrawal. This sets up a recurring cycle of T cell differentiation (Naive→TEFF→TSCM→TCM→TEM→TEFF) that would result in an oscillating — on–off–on or off–on–off — pattern of transcriptional and epigenetic changes over time. b | In the developmental, or linear, differentiation model, the progressive acquisition of effector function during CD8+ T cell differentiation (Naive→TEFF→TSCM→TCM→TEM→TEFF) depends on the strength and duration of antigenic signalling and results in the gradual loss of memory-associated gene expression and gain of effector-associated gene expression. These transcriptional changes are accompanied by similar changes in the epigenetic landscape, which are illustrated by the gradual, or progressive, gain or loss of activating and repressive histone modifications. TCM, central memory T; TEM, effector memory T; TSCM, stem cell memory T.
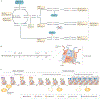
Figure 2 |. Features of DNA methylation and histone modifications.
a | The DNA methylation cycle of cytosine nucleotide bases is depicted. DNA (cyto-sine-5)-methyltransferase 3A (DNMT3A) and DNMT3B add methylation (m) modifications to unmodified cytosines in a de novo manner, whereas DNMT1 acts to maintain established patterns during DNA replication. Passive demethylation results from a lack of maintenance methylation and is replication dependent, whereas active demethylation is directed by Ten-eleven translocation (TET) proteins and can be either replication dependent or independent. TET proteins mediate the serial oxidation of methylated cytosines (red bases), resulting in multiple intermediary bases, including 5-hydroxymethylcytosine (green bases). Eventually, modified bases are returned to an unmodified state via the base excision repair (BER) pathway. b | Nucleosomes consist of two copies each of the histone proteins H2A, H2B, H3 and H4 encircled by DNA. The amino-terminal tails of histone proteins protrude from the nucleosome structure and can be post-translationally modified. The amino acid sequence of the histone H3 N-terminal tail is shown along with the position of select lysines (K) that are subject to methylation (me) and/or acetylation (ac); however, this is not an exhaustive list of all H3 post-translational modifications. c | Chromatin can be broadly categorized as either euchromatin or heterochromatin on the basis of its accessibility level. Euchromatin is characterized by an open chromatin conformation that is less compact and more accessible by regulatory proteins (yellow oval). Heterochromatin is categorized as either facultative or constitutive. Both are more compact and inaccessible relative to euchromatin; however, constitutive heterochromatin is generally more compacted and is associated with gene-poor repetitive regions that largely remain in a closed state. Facultative heterochromatin, by contrast, is associated with regions that often transition to an open conformation as transcriptional requirements change. Each chromatin state has specific histone post-translational modifications that are associated with it, as depicted.
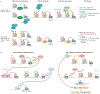
Figure 3 |. Mechanisms of epigenetic-mediated control of CD8 + T cell differentiation.
a | Repressive histone-modifying enzymes Polycomb complex protein BMI1 and histone-lysine _N_-methyltransferase EZH2, as part of Polycomb repressive complex 1 (PRC1) and PRC2, respectively, contribute to the functional phenotypes of memory-like killer cell lectin-like subfamily G member 1 (KLRG1)–CD8+ T cells and terminally differentiated KLRG1+CD8+ T cells via subset-specific activity. Differential targeting of Notch repressors Numb and Fbxw7 and cell cycle repressors, including Cdkn2a, results in activation or repression of Notch and retinoblastoma-associated protein 1 (RB1)–p53 pathways, ultimately affecting polyfunctionality and apoptosis. Similarly, differential repression of memory-associated loci affects the memory-associated transcriptional programme. In memory-like subsets, active transcription facilitated by forkhead box protein O1 (FOXO1) binding may inhibit EZH2-mediated repression. b | The left panel illustrates a bromodomain-containing protein 4 (BRD4)-dependent regulatory cascade that is critical for normal effector cell differentiation. The recognition of lysine acetylation marks (yellow pentagon) by the histone reader protein BRD4 increases expression of the transcription factor Batf (which encodes basic leucine zipper transcriptional factor ATF-like (BATF)). BATF, together with activator protein 1 (AP-1), transcriptionally represses the histone deacetylase gene Sirt1 (which encodes protein deacetylase sirtuin 1 (SIRT1)). Decreased activity of SIRT1 increases acetylation at the Tbx21 (which encodes T-bet) locus, resulting in increased expression of Tbx21 and T-bet target genes. The addition of the bromodomain inhibitor JQ1 upends this pathway (right panel), with the lack of Sirt1 repression contributing to decreased histone acetylation and the reduced expression of Tbx21 and T-bet targets. Ultimately, JQ1 inhibits effector differentiation, which results in increased stem cell memory T (TSCM) and central memory T (TCM) cells. Ac, acetylation; TF, transcription factor.
Figure 4 |. The arrested model of CD8 + T cell exhaustion.
The arrested model of CD8+ T cell exhaustion represents a branchpoint of the linear differentiation model, at which strong and/or repetitive antigenic stimulation, often accompanied by a lack of co-stimulatory signals, arrests canonical differentiation. Increasingly, it has been shown that the exhausted state is heterogeneous, with a subset of exhausted T (TEX) cells exhibiting memory-like phenotypes and characterized by specific cell surface markers (left side, light orange box). Conversely, more differentiated TEX cells exhibit their own unique cell surface marker expression (right side, dark orange box). The stage along canonical T cell differentiation at which cells become arrested may determine their TEX cell phenotype (light blue arrows); however, continued differentiation within the exhausted state may also occur. Memory-like TEX cells appear to be more responsive to checkpoint inhibitor therapy, which we hypothesize releases arrested cells, returning them to the canonical differentiation path. Importantly, in the linear model, this would ultimately result in terminal differentiation and apoptosis of TEX cells that had reversed the arrested state. CXCR5, CXC-chemokine receptor 5; EOMES, eomesodermin homologue; PD1, programmed cell death protein 1; TCF1, transcription factor 1; TIM3, T cell immunoglobulin mucin receptor 3; TM, memory T; TEFF, effector T.
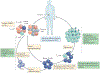
Figure 5 |. Interventions for improving clinical outcomes of adoptive cell therapy.
Currently, T cells used for adoptive cell therapy (ACT) are either obtained directly from tumours in the form of tumour-infiltrating lymphocytes (TILs) or isolated from patient peripheral blood mononuclear cells (PBMCs). These cells are tested for tumour reactivity or transduced with a tumour-reactive chimeric antigen receptor (CAR) or T cell receptor (TCR) followed by an extensive ex vivo expansion step before reinfusion into the patient. Although clinically effective in some cases, there are major challenges associated with current protocols. In both the starting T cell population and the population obtained following expansion, cells exhibit a more differentiated and/or exhausted phenotype, which may hinder in vivo effectiveness. Additionally, following transfer, cells will encounter an immunosuppressive tumour microenvironment that may further promote exhaustion. There are many potential fixes for the challenges currently affecting ACT. Cellular reprogramming of PBMCs or TILs would obtain a younger, less differentiated starting population, and pharmacological interventions to target relevant signalling pathways and/or epigenetic modifying proteins could allow for T cell expansion without differentiation. Additionally, T cells could be genetically manipulated to remain impervious to the exhaustion-inducing effects of the tumour microenvironment. Targeting non-coding regulatory regions is of therapeutic interest for its ability to alter gene expression in a subset-specific manner. These potential solutions will require a thorough understanding of the proteins and epigenetic landscapes regulating the differentiation process. iPSCs, induced pluripotent stem cells; TEFF, effector T; TEX, exhausted T; TM, memory T.
Similar articles
- Global DNA methylation remodeling accompanies CD8 T cell effector function.
Scharer CD, Barwick BG, Youngblood BA, Ahmed R, Boss JM. Scharer CD, et al. J Immunol. 2013 Sep 15;191(6):3419-29. doi: 10.4049/jimmunol.1301395. Epub 2013 Aug 16. J Immunol. 2013. PMID: 23956425 Free PMC article. - Generating long-lived CD8(+) T-cell memory: Insights from epigenetic programs.
Dogra P, Ghoneim HE, Abdelsamed HA, Youngblood B. Dogra P, et al. Eur J Immunol. 2016 Jul;46(7):1548-62. doi: 10.1002/eji.201545550. Eur J Immunol. 2016. PMID: 27230488 Free PMC article. Review. - Lineage relationship of CD8(+) T cell subsets is revealed by progressive changes in the epigenetic landscape.
Crompton JG, Narayanan M, Cuddapah S, Roychoudhuri R, Ji Y, Yang W, Patel SJ, Sukumar M, Palmer DC, Peng W, Wang E, Marincola FM, Klebanoff CA, Zhao K, Tsang JS, Gattinoni L, Restifo NP. Crompton JG, et al. Cell Mol Immunol. 2016 Jul;13(4):502-13. doi: 10.1038/cmi.2015.32. Epub 2015 Apr 27. Cell Mol Immunol. 2016. PMID: 25914936 Free PMC article. - Epigenetic remodeling of the IL-2 and IFN-gamma loci in memory CD8 T cells is influenced by CD4 T cells.
Northrop JK, Thomas RM, Wells AD, Shen H. Northrop JK, et al. J Immunol. 2006 Jul 15;177(2):1062-9. doi: 10.4049/jimmunol.177.2.1062. J Immunol. 2006. PMID: 16818762 - Transcriptional and Epigenetic Regulation of Effector and Memory CD8 T Cell Differentiation.
Chen Y, Zander R, Khatun A, Schauder DM, Cui W. Chen Y, et al. Front Immunol. 2018 Dec 7;9:2826. doi: 10.3389/fimmu.2018.02826. eCollection 2018. Front Immunol. 2018. PMID: 30581433 Free PMC article. Review.
Cited by
- YTHDF2 upregulation and subcellular localization dictate CD8 T cell polyfunctionality in anti-tumor immunity.
Zhang H, Luo X, Yang W, Wu Z, Zhao Z, Pei X, Zhang X, Chen C, Lei JH, Shi Q, Zhao Q, Chen Y, Wu W, Zeng Z, Ju HQ, Qiu M, Liu J, Shen B, Chen M, Chen J, Deng CX, Xu RH, Hou J. Zhang H, et al. Nat Commun. 2024 Nov 5;15(1):9559. doi: 10.1038/s41467-024-53997-6. Nat Commun. 2024. PMID: 39500904 Free PMC article. - Viscoelastic synthetic antigen-presenting cells for augmenting the potency of cancer therapies.
Liu Z, Li YR, Yang Y, Zhu Y, Yuan W, Hoffman T, Wu Y, Zhu E, Zarubova J, Shen J, Nan H, Yeh KW, Hasani-Sadrabadi MM, Zhu Y, Fang Y, Ge X, Li Z, Soto J, Hsiai T, Yang L, Li S. Liu Z, et al. Nat Biomed Eng. 2024 Oct 25. doi: 10.1038/s41551-024-01272-w. Online ahead of print. Nat Biomed Eng. 2024. PMID: 39455719 - Immunotherapy for Treatment of Pleural Mesothelioma: Current and Emerging Therapeutic Strategies.
Chiec L, Bruno DS. Chiec L, et al. Int J Mol Sci. 2024 Oct 9;25(19):10861. doi: 10.3390/ijms251910861. Int J Mol Sci. 2024. PMID: 39409190 Free PMC article. Review. - SATB1 prevents immune cell infiltration by regulating chromatin organization and gene expression of a chemokine gene cluster in T cells.
Wang B, Bian Q. Wang B, et al. Commun Biol. 2024 Oct 11;7(1):1304. doi: 10.1038/s42003-024-07021-8. Commun Biol. 2024. PMID: 39394451 Free PMC article. - Systems immunology approaches to study T cells in health and disease.
Yang A, Poholek AC. Yang A, et al. NPJ Syst Biol Appl. 2024 Oct 9;10(1):117. doi: 10.1038/s41540-024-00446-1. NPJ Syst Biol Appl. 2024. PMID: 39384819 Free PMC article. Review.
References
- Opferman JT, Ober BT & Ashton-Rickardt PG Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science 283, 1745–1748 (1999). - PubMed
Publication types
MeSH terms
Grants and funding
- BB/N007794/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- Z01 BC010763-03/ImNIH/Intramural NIH HHS/United States
- Z01 BC010763/ImNIH/Intramural NIH HHS/United States
- BBS/E/B/000C0409/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 22597/CRUK_/Cancer Research UK/United Kingdom
- BBS/E/B/000C0407/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials