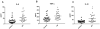High complement levels in astrocyte-derived exosomes of Alzheimer disease - PubMed (original) (raw)
. 2018 Mar;83(3):544-552.
doi: 10.1002/ana.25172. Epub 2018 Mar 10.
Affiliations
- PMID: 29406582
- PMCID: PMC5867263
- DOI: 10.1002/ana.25172
High complement levels in astrocyte-derived exosomes of Alzheimer disease
Edward J Goetzl et al. Ann Neurol. 2018 Mar.
Abstract
Objective: Astrocytes fulfill neuronal trophic roles normally, but are transformed in Alzheimer disease (AD) into A1-type reactive astrocytes that may destroy neurons through unknown mechanisms.
Methods: To investigate astrocyte inflammatory mechanisms, astrocyte-derived exosomes (ADEs) were isolated immunochemically from plasma samples of AD patients and matched controls for enzyme-linked immunosorbent assay quantification of complement proteins.
Results: ADE levels of C1q, C4b, C3d, factor B, factor D, Bb, C3b, and C5b-C9 terminal complement complex, but not mannose-binding lectin, normalized by the CD81 exosome marker were significantly higher for AD patients (n = 28) than age- and gender-matched controls (all p < 0.0001). ADE normalized levels of interleukin (IL)-6, tumor necrosis factor-α, and IL-1β were significantly higher for AD patients than controls, but there was greater overlap between the two groups than for complement proteins. Mean ADE levels of complement proteins for AD patients in a longitudinal study were significantly higher (n = 16, p < 0.0001) at the AD2 stage of moderate dementia than at the AD1 preclinical stage 5 to 12 years earlier, which were the same as for controls. ADE levels of complement regulatory proteins CD59, CD46, decay-accelerating factor (DAF), and complement receptor type 1, but not factor I, were significantly lower for AD patients than controls (p < 0.0001 for CD59 and DAF), were diminished by the AD1 stage, and were further decreased at the AD2 stage.
Interpretation: ADE complement effector proteins in AD are produced by dysregulated systems, attain higher levels than in controls, and may potentially damage neurons in the late inflammatory phase of AD. Ann Neurol 2018;83:544-552.
© 2018 American Neurological Association.
Conflict of interest statement
Potential Conflicts of Interest: E.J.G. has filed an application with the U.S. Patent Office for the platform and methodologies described in this report. The remaining authors declare no potential conflicts of interest.
Figures
Figure 1. ADE levels of inflammatory cytokines in cross-sectional control and AD groups
Each point represents the value for a control subject or AD patient and the horizontal line in point clusters is the mean level for that group. Mean±S.E.M. control and AD patient values, respectively, are 27.4±4.22 pg/ml and 55.5±6.02 pg/ml for IL-6, 158±16.2 pg/ml and 226±17.9 pg/ml for TNF-α, and 29.6±2.63 pg/ml and 61.3±7.75 pg/ml for IL-1β. The significance of differences between values for controls and AD patients was calculated by an unpaired Student's t test; +=p<0.01 and *=p<0.001.
Figure 2. ADE levels of complement effector proteins in cross-sectional control and AD groups
Each point represents the value for a control subject or AD patient and the horizontal line in point clusters is the mean level for that group. Mean±S.E.M. control and AD patient values, respectively, are 13,902±1105 pg/ml and 48,906±2528 for C1q, 64,305±4408 pg/ml and 166,151±7647 pg/ml for C4b, 48,705±6,966 pg/ml and 416,093±44,866 pg/ml for C3d, 113,321±14,402 pg/ml and 547,292±66,082 pg/ml for complement factor B, 1465±140 pg/ml and 5800±658 pg/ml for complement factor D, 91,933±10,641 pg/ml and 252,271±11,912 pg/ml for factor B fragment Bb, 25,175±1973 pg/ml and 80,703±4286 pg/ml for C3b, 355±27.1 pg/ml and 1212±109 pg/ml for C5b-C9 TCC, and 874±82.1 pg/ml and 1141±128 pg/ml for MBL. The significance of differences between values for controls and AD patients was calculated by an unpaired Student's t test; **=p<0.0001. For comparison, NDE values were 68.8±7.09 pg/ml, 11,562±987 pg/ml, 9,354±602 pg/ml, 14,624±835 pg/ml, 7,128±840 pg/ml and 306±29.1 pg/ml for C5b-C9 TCC, C3d, C4b, Bb, factor B and factor D, respectively, in AD patients and were 54.2±6.37 pg/ml, 1,855±134 pg/ml, 3,851±276 pg/ml, 6,029±438 pg/ml, 2,055±276 pg/ml and 94.2±10.7 pg/ml for C5b-C9 TCC, C3d, C4b, Bb, and factors B and D, respectively, in controls (Table 2).
Figure 3. ADE levels of complement regulatory proteins in cross-sectional control and AD groups
Each point represents the value for a control subject or AD patient and the horizontal line in point clusters is the mean level for that group. Mean±S.E.M. control and AD patient values, respectively, are 1211±68.5 pg/ml and 398±37.1 pg/ml for CD59, 57.2±4.39 pg/ml and 36.1±2.47 pg/ml for CD46, 447±31.4 pg/ml and 336±26.1 pg/ml for CR1, 35,197±3735 pg/ml and 4,563±654 pg/ml for DAF, and 6281±562 pg/ml and 5010±346 pg/ml for factor I. The significance of differences between values for controls and AD patients was calculated by an unpaired Student's t test; *= p<0.01 and **=p<0.0001.
Figure 4. ADE levels of complement proteins and cytokines in the longitudinal study
Each point represents the value for a control subject or AD patient and the horizontal line in point clusters is the mean level for that group. Control, AD1 patient and AD2 patient values (mean±S.E.M.), respectively, are 63,621±3056, 63,901±3130 and 163,273±4864 for C4b, 62,958±8,945, 86,911±10,020 and 215,660±23,018 pg/ml for C3d, 82,681±3,921, 84,385±12,808 and 548,930±77,855 pg/ml for complement factor B, 85,716±6181, 85,123±4836 and 247,574± 10,740 pg/ml for factor B fragment Bb, 24,347±2350, 22,212±1866 and 70,039±5245 pg/ml for C3b, 400±43.4, 359±31.7 and 832±87.2 pg/ml for C5b-C9 TCC; 1272±61.4, 757±33.6 and 409±15.4 pg/ml for CD59, and 32,123±1733, 13,352±803 and 4369+320 pg/ml for DAF. The significance of differences between values for controls and AD1 patients was calculated by an unpaired Student's t test and for differences between values for AD1 and AD2 patients was calculated by a paired Student's t test; **=p<0.0001.
Similar articles
- Traumatic brain injury increases plasma astrocyte-derived exosome levels of neurotoxic complement proteins.
Goetzl EJ, Yaffe K, Peltz CB, Ledreux A, Gorgens K, Davidson B, Granholm AC, Mustapic M, Kapogiannis D, Tweedie D, Greig NH. Goetzl EJ, et al. FASEB J. 2020 Feb;34(2):3359-3366. doi: 10.1096/fj.201902842R. Epub 2020 Jan 8. FASEB J. 2020. PMID: 31916313 Free PMC article. - Deficient neurotrophic factors of CSPG4-type neural cell exosomes in Alzheimer disease.
Goetzl EJ, Nogueras-Ortiz C, Mustapic M, Mullins RJ, Abner EL, Schwartz JB, Kapogiannis D. Goetzl EJ, et al. FASEB J. 2019 Jan;33(1):231-238. doi: 10.1096/fj.201801001. Epub 2018 Jun 20. FASEB J. 2019. PMID: 29924942 Free PMC article. - Complement protein levels in serum astrocyte-derived exosomes are associated with cognitive impairment in obstructive sleep apnea.
Li M, Sun C, Xue S, Leng B, Sun H, Shen T, Liu X, Li Z, Shang X, Zhang J. Li M, et al. J Clin Sleep Med. 2023 Apr 1;19(4):727-739. doi: 10.5664/jcsm.10412. J Clin Sleep Med. 2023. PMID: 36692174 Free PMC article. - The Dual Role of Astrocyte-Derived Exosomes and Their Contents in the Process of Alzheimer's Disease.
Liu Z, Zhang H, Liu S, Hou Y, Chi G. Liu Z, et al. J Alzheimers Dis. 2023;91(1):33-42. doi: 10.3233/JAD-220698. J Alzheimers Dis. 2023. PMID: 36373321 Review. - [Cell-associated complement regulatory proteins and their relation to disease processes].
Seya T. Seya T. Rinsho Byori. 1991 Dec;39(12):1317-24. Rinsho Byori. 1991. PMID: 1723433 Review. Japanese.
Cited by
- The Importance of Complement-Mediated Immune Signaling in Alzheimer's Disease Pathogenesis.
Batista AF, Khan KA, Papavergi MT, Lemere CA. Batista AF, et al. Int J Mol Sci. 2024 Jan 9;25(2):817. doi: 10.3390/ijms25020817. Int J Mol Sci. 2024. PMID: 38255891 Free PMC article. Review. - Advancing Alzheimer's Therapeutics: Exploring the Impact of Physical Exercise in Animal Models and Patients.
Andrade-Guerrero J, Rodríguez-Arellano P, Barron-Leon N, Orta-Salazar E, Ledesma-Alonso C, Díaz-Cintra S, Soto-Rojas LO. Andrade-Guerrero J, et al. Cells. 2023 Oct 27;12(21):2531. doi: 10.3390/cells12212531. Cells. 2023. PMID: 37947609 Free PMC article. Review. - Neuronal and Astrocytic Extracellular Vesicle Biomarkers in Blood Reflect Brain Pathology in Mouse Models of Alzheimer's Disease.
Delgado-Peraza F, Nogueras-Ortiz CJ, Volpert O, Liu D, Goetzl EJ, Mattson MP, Greig NH, Eitan E, Kapogiannis D. Delgado-Peraza F, et al. Cells. 2021 Apr 23;10(5):993. doi: 10.3390/cells10050993. Cells. 2021. PMID: 33922642 Free PMC article. - Extracellular Vesicles From 3xTg-AD Mouse and Alzheimer's Disease Patient Astrocytes Impair Neuroglial and Vascular Components.
González-Molina LA, Villar-Vesga J, Henao-Restrepo J, Villegas A, Lopera F, Cardona-Gómez GP, Posada-Duque R. González-Molina LA, et al. Front Aging Neurosci. 2021 Feb 19;13:593927. doi: 10.3389/fnagi.2021.593927. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33679370 Free PMC article. - Emerging Role of Extracellular Vesicles in Intercellular Communication in the Brain: Implications for Neurodegenerative Diseases and Therapeutics.
Sarkar S, Patranabis S. Sarkar S, et al. Cell Biochem Biophys. 2024 Jun;82(2):379-398. doi: 10.1007/s12013-024-01221-z. Epub 2024 Feb 1. Cell Biochem Biophys. 2024. PMID: 38300375 Review.
References
- Liddelow SA, Barres BA. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity. 2017 Jun 20;46(6):957–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous