Clathrin coat controls synaptic vesicle acidification by blocking vacuolar ATPase activity - PubMed (original) (raw)
Clathrin coat controls synaptic vesicle acidification by blocking vacuolar ATPase activity
Zohreh Farsi et al. Elife. 2018.
Abstract
Newly-formed synaptic vesicles (SVs) are rapidly acidified by vacuolar adenosine triphosphatases (vATPases), generating a proton electrochemical gradient that drives neurotransmitter loading. Clathrin-mediated endocytosis is needed for the formation of new SVs, yet it is unclear when endocytosed vesicles acidify and refill at the synapse. Here, we isolated clathrin-coated vesicles (CCVs) from mouse brain to measure their acidification directly at the single vesicle level. We observed that the ATP-induced acidification of CCVs was strikingly reduced in comparison to SVs. Remarkably, when the coat was removed from CCVs, uncoated vesicles regained ATP-dependent acidification, demonstrating that CCVs contain the functional vATPase, yet its function is inhibited by the clathrin coat. Considering the known structures of the vATPase and clathrin coat, we propose a model in which the formation of the coat surrounds the vATPase and blocks its activity. Such inhibition is likely fundamental for the proper timing of SV refilling.
Keywords: E. coli; acidification; clathrin coat; endocytosis; human; mouse; neuroscience; proton pump; synaptic vesicle; vATPase.
© 2018, Farsi et al.
Conflict of interest statement
ZF, SG, MK, BR, AW, EL, CM, IM No competing interests declared, RJ Reviewing editor, eLife
Figures
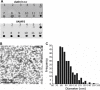
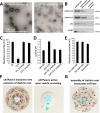
Figure 1.. Isolation of clathrin-coated vesicles from mouse brain.
(A) Schema illustrating the isolation procedure of CCVs from mouse brains. Numbers 1 and 2 represent steps where Ficol and sucrose (at final conc. of 6.25 (wt/v)) were added to the sample, and where the sample was diluted 5x with buffer, respectively. See Suppl. Data for details. (B) Electron micrograph of isolated CCVs after negative staining. (C) Immunoblots of fractions collected during the CCV isolation protocol for various marker proteins (proteins were separated at the 4–15% gradient gel and detected by the Li-COR Odyssey imaging system). See also Supplementary file 1 for mass spectrometry analysis of SVs and CCVs samples. (D) Reconstructed CCV from 6114 particles sized up to a diameter of 80 nm, with a D6 symmetry imposed, shows that ex vivo CCVs adopt the same ‘barrel-like’ structure as previously reported, and reveal the position of vesicle in its center (see also Suppl. Data and Figure 1—figure supplement 2). The median values extracted from the reconstruction of clathrin coat and vesicle within the coat are (note that the structure is a barrel): coated vesicle diameter 75 nm x 73.5 nm, coat thickness 15 nm, vesicle diameter 40 × 35 nm.
Figure 1—figure supplement 1.. Optimization of a procedure for the CCV isolation from mouse brains, and characterization of CCV size.
(A) Enrichment of CCVs in lower fraction of the sucrose gradient, as detected by clathrin LC and VAMP2 immunoblotting. (B) Electron micrograph of isolated CCVs after negative staining. (C) Histogram representing the distribution of CCV sizes (in nm).
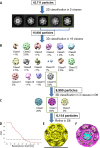
Figure 1—figure supplement 2.. Determining the quality and the properties of clathrin coat of CCVs isolated from mouse brains by cryo-EM.
(A–C) Reconstruction scheme for CCVs isolated from mouse brains by cryo-EM in RELION. (A) 43,711 particles were manually picked and classified into five 2D classes in RELION. The distribution of five classes was as follows: (1) 19.7% (distance 79.3 nm); (2) 17% (distance 62.7 nm); (3) 21.8% (distance 72,5); (4) 26.8% (distance 64 nm); and (5) 14.7% (distance 72.8 nm). From those five classes, we have selected two most abundant classes (classes 3 and 4) for the further processing (due to particle abundance, quality and sizes comparable to the size of previously reported ‘barrel-like’ clathrin coat). The length of each image is 123.5 nm. (B) The selected particles were classified into sixteen 3D classes without any symmetry (=C1) enforced. Of resulting structures, class 16 has the highest population among all classes, and it also exhibits a symmetry that resembles D6. Therefore, we continued with the class #16. (C) Re-classification in 3D of the ‘class #16’ particles from the previous classification. D6 was enforced in the classification this time. We continued again with the highest populated class (class #2). (D–E) The particles from the class 2 of the previous run were chosen for the refinement to calculate the final structure (shown in E). (D) Fourier Shell Correlation (FSC) for the final reconstruction. For the 0.143 Gold Standard FSC criterion (dotted line), the calculated resolution is ~26 Å. (F) The reconstructed particle (shown in purple; deposited with the EMDB-ID #4335) was comparable in size and symmetry to the ‘barrel-like’ empty clathrin cages reported in Fotin et al. (2004) (shown in yellow in F; EMDB-ID 5119).
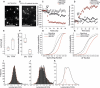
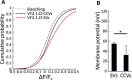
Figure 2.. Measurement of the electrochemical gradient in SVs and CCVs.
(A–B) Representative images of single (A) spH-CCVs and (B) VF2.1.Cl-labeled CCVs using TIRF microscopy. (C–D) Averaged fluorescence traces of single (C) spH-SV and spH-CCVs, and (D) VF2.1.Cl-labeled SVs and VF2.1.Cl-labeled CCVs over time in response to ATP. The control traces indicate the fluorescence response of the spH-CCVs or VF2.1.Cl-labeled CCVs over experimental timescale without ATP addition (the same traces were obtained for spH-SVs and VF2.1.Cl-labeled SVs). Error bars indicate SD from more than 1000 vesicles compiled from 4 to 7 experimental replicates. (E) Box plot representation of luminal pH of single SVs and CCVs after addition of 1 mM ATP (box: 1 st and 3rd quartile, line: median, whiskers: the minimum and maximum values). Note that the luminal pH of vesicles equilibrates to 7.4 as shown in Farsi et al. (2016). (F) Box plot representation of membrane potential of single SVs and CCVs after addition of 3 mM ATP. (G–H) Cumulative frequency plots generated from fluorescence change associated with ATP addition in (G) spH-SVs and spH-CCVs, and (H) VF2.1.Cl-labeled SVs and VF2.1.Cl-labeled CCVs. The dotted line indicates the fluorescence response of the probes over experimental timescale without ATP addition. (I–J) Histograms representing the luminal pH of (I) spH-SVs (n = 3,625) and (J) spH-CCVs (n = 2,233) upon addition of 1 mM ATP. Red lines indicate single and two-Gaussian models to SV and CCV populations, respectively. (K) The population of vesicles contributing to the lower pH (likely CCVs with damaged coat and/or very few SVs) in the CCV population consists of about 6% of the total vesicles measured.
Figure 2—figure supplement 1.. Flow chart of the single vesicle assay for measuring pH and membrane potential of SVs and CCVs.
Isolated SVs and CCVs were separately immobilized on glass coverslips and imaged with a TIRF setup. The fluorescence change of the immobilized vesicles in response to ATP was measured and compared between two samples.
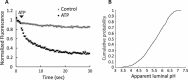
Figure 2—figure supplement 2.. Full acidification of spH-SVs to low luminal pH.
(A) Addition of 3 mM ATP in the presence of 30 mM chloride led to almost 80% quenching of fluorescence in single spH-SVs (black trace). The control trace shows the fluorescence change of spH-SVs in the absence of ATP, indicating that photobleaching was insignificant over experimental timescale. Error bars indicate SEM of more than 500 spH-SVs. (B) Cumulative curve generated from apparent luminal pH of more than 500 spH-SVs at steady state after addition of 3 mM ATP. The average luminal pH of spH-SVs after correction for bleaching was 5.82 ± 0.03 (SEM) indicating full acidification of vesicles in the presence of chloride (Miesenböck et al., 1998).
Figure 2—figure supplement 3.. Impairment of acidification in CCVs in the presence of chloride.
(A) Cumulative curve generated from ∆F/F0 in VF2.1.Cl labeled CCVs and SVs associated with addition of 3 mM ATP in the presence of 20 mM TEA.Cl in the bath solution. The control trace shows the fluorescence change of VF2.1.Cl-labeled CCVs over experimental timescale without ATP addition (the same trace was obtained for spH-SVs and VF2.1.Cl-labeled SVs) (B) Averaged membrane potential in single VF2.1.Cl-labeled CCVs and SVs upon addition of 3 mM ATP in the presence of 20 mM TEA.Cl in the bath solution. As expected, smaller membrane potential was formed across the membrane of SVs in the presence of chloride (∆ψ = 55.02 ± 2.63 (SD)) in response to 3 mM ATP. However, the magnitude of ∆ψ was significantly smaller in CCVs (p=0.04), indicating that the impairment of acidification in CCVs shown in Figure 2 is not due to the absence of chloride. Error bars indicate SD of 4 experimental replicates.
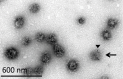
Figure 2—figure supplement 4.. EM image of a CCV with a damaged coat.
A CCV with a damaged coat is marked with a black arrow. We noticed that upon immuno-gold labeling against clathrin heavy chain (black arrowhead), the antibody can recognize better the vesicles whose coat was damaged, possibly due to better availability of the epitope in a partially disassembled vesicles.
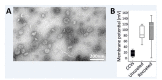
Figure 3.. Functional analysis of the vATPase on CCVs.
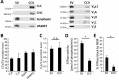
(A) Immunoblots of isolated SVs and CCVs for the clathrin light (LC) and heavy chains (HC), and SV marker proteins synaptophysin (Syph) and VAMP2 (left panel), as well as various Vo and V1 subunits of vATPase (right panel). (B) The ratio of the Vo and V1 as well as synaptophysin and VAMP2 (as SV markers) detected by immunoblotting in equal protein amount of CCVs and SVs (C) Normalized levels of Vo and V1 in CCV and SV samples, indicating that Vo:V1 ratio is 1:1 in both preparations. (D) ATPase activity measured in 1.3 µg of isolated SVs and CCVs. (E) Blocking percentage of ATPase activity by NEM (vATPase inhibitor) in 1.3 µg of SVs and CCVs. Error bars in (B–E) represent SD of 3–4 experimental replicates (p<0.01 for D and E, and >0.05 for C).
Figure 3—figure supplement 1.. Both Vo and V1 subunits are present on CCVs.
Immunoblot for Voa and V1A subunits indicate presence of subunits during the CCV isolation procedure.
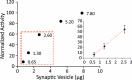
Figure 3—figure supplement 2.. ATPase activity measurements in isolated SVs.
To make sure that the measured ATPase activity is within the standard curve range of the used kit, we tested different amounts of isolated SVs. The measurements of ATPase activity were then performed with 1.3 µg of SVs and CCVs which is in the liner range (inset).
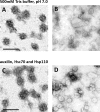
Figure 4.. CCV uncoating revealed that the vATPase is blocked by clathrin coat.
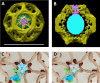
(A) Electron micrographs of negatively stained CCVs before and after uncoating with 300 mM Tris-buffer pH 9.0. (B) Western blot of CCVs, uncoated vesicles and supernatant after uncoating shows dissociation of clathrin LC and HC, as well as AP180 and AP2, from the uncoated vesicle (proteins were separated at the 10% gel and detected by chemiluminescence). (C–D) Membrane potential (C) and luminal pH (D) of acidified SVs and CCVs before and after treatment with Tris-buffer (pH 9.0). Error bars represent SD of 3–4 experimental replicates done on over 1000 vesicles each. (E) Membrane potential of acidified CCVs after treatment with 300 mM Tris-buffer (pH 9.0), 500 mM Tris buffer (pH 7.0) and ‘enzymatic’ treatment with 1.7 µg auxilin, 4.8 µg Hsc70 and 1.7 µg Hsp110 proteins. Error bars represent SD of 3 experimental replicates done on over 1000 vesicles each. (F–G) Model of vATPase block by clathin coat: solved structures of vATPase, clathrin tripods and AP2 complex were used to check how vATPase fits within the clathrin lattice. The plasma membrane is depicted in light beige, clathrin triskelia in dark beige/brown; vATPase complex in gray (when inactive), light blue (when active) and dark green (V1H-subunit); AP2 complex in purple/blue/light green. As clathrin triskelia are recruited (through AP2), clathrin ring starts building around the vATPase complex. Insertion of the last triskelion of the clathrin ring would collide with the regulatory V1H-subunit of vATPase (G), thus we hypothesize that the displacement of regulatory V1H-subunit inwards results in the block of the vATPase activity. For more details, see Suppl. Data.
Figure 4—figure supplement 1.. CCV uncoating revealed that the vATPase is blocked by clathrin coat.
(A–B) Electron micrographs of negatively stained CCVs before (A) and after (B) uncoating with neutral 500 mM Tris-buffer (pH 7.0). (C–D) Electron micrographs of negatively stained CCVs before (C) and after (D) uncoating with auxilin, Hsc70 and Hsp110. For experimental details, see Methods. Scale bar 100 nm.
Figure 4—figure supplement 2.. Proposed model of vATPase block by clathin coat.
(A–B) Molecular modelling experiments where the cryo-EM structure of the vATPase complex (from Zhao et al., 2015) was docked into our cryo-EM structure of CCV (A top view, the coat is made slightly transparent; B cross-section through the reconstructed CCV – for details see Figure 2—figure supplement 3). (C–D) Solved structures of vATPase, clathrin tripods and AP2 were used to show how vATPase may fit within the clathrin lattice. The plasma membrane is depicted in light beige, clathrin triskelia in dark beige/brown; vATPase complex in gray (when inactive), light blue (when active) and dark green (V1H-subunit); AP2 complex in purple/dark blue/light green. (C) As clathrin triskelia are recruited (through AP2), clathrin ring starts building around the vATPase complex. (D) Insertion of the last clathrin triskelion of clathrin ring would collide with the regulatory V1H-subunit of vATPase, thus we hypothesize that the displacement of regulatory V1H-subunit inwards, in order to accommodate the vATPase complex into the hexagon of the CCV lattice. For more details, see Supplementary Movies.
Author response image 1.. Partial recoating of previously uncoated CCVs was not robust enough to produce a significant difference in acidification assay.
(A) Electron micrographs of negatively stained sample after the vernight “recoating” treatment as detailed in Materials and methods. While coat formation sometimes occurs without the vesicle at its center, several vesicles surrounded by a new coat are clearly seen (indicated by a white arrow). Scale bar 200 nm. (B) Membrane potential of acidified vesicles after the overnight “recoating” treatment Error bars represent SD of 3 experimental replicates.
Author response image 2.. Cryo-electron tomography on partially uncoated CCVs reveals that clathrin coat-vATPase interactions exist.
(A) Electron micrographs of partially uncoated CCVs (in tightly packed CCVs, vATPase could not be detected). (B) Zoomed image of two vesicles at different views, as indicated. Red arrowhead points presumably to vATPase, blue arrowheads to clathrin filaments. See cryo-electron tomography Video for a better view.
Similar articles
- Regulation of synaptic vesicle acidification at the neuronal synapse.
Gowrisankaran S, Milosevic I. Gowrisankaran S, et al. IUBMB Life. 2020 Apr;72(4):568-576. doi: 10.1002/iub.2235. Epub 2020 Jan 25. IUBMB Life. 2020. PMID: 31981303 Review. - Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling.
Blondeau F, Ritter B, Allaire PD, Wasiak S, Girard M, Hussain NK, Angers A, Legendre-Guillemin V, Roy L, Boismenu D, Kearney RE, Bell AW, Bergeron JJ, McPherson PS. Blondeau F, et al. Proc Natl Acad Sci U S A. 2004 Mar 16;101(11):3833-8. doi: 10.1073/pnas.0308186101. Epub 2004 Mar 8. Proc Natl Acad Sci U S A. 2004. PMID: 15007177 Free PMC article. - Glutamate uptake occurs at an early stage of synaptic vesicle recycling.
Prior IA, Clague MJ. Prior IA, et al. Curr Biol. 1997 May 1;7(5):353-6. doi: 10.1016/s0960-9822(06)00159-x. Curr Biol. 1997. PMID: 9115399 - A human genome-wide screen for regulators of clathrin-coated vesicle formation reveals an unexpected role for the V-ATPase.
Kozik P, Hodson NA, Sahlender DA, Simecek N, Soromani C, Wu J, Collinson LM, Robinson MS. Kozik P, et al. Nat Cell Biol. 2013 Jan;15(1):50-60. doi: 10.1038/ncb2652. Nat Cell Biol. 2013. PMID: 23263279 Free PMC article. - Structure, subunit function and regulation of the coated vesicle and yeast vacuolar (H(+))-ATPases.
Arata Y, Nishi T, Kawasaki-Nishi S, Shao E, Wilkens S, Forgac M. Arata Y, et al. Biochim Biophys Acta. 2002 Sep 10;1555(1-3):71-4. doi: 10.1016/s0005-2728(02)00257-8. Biochim Biophys Acta. 2002. PMID: 12206894 Review.
Cited by
- Neurodevelopmental and synaptic defects in DNAJC6 parkinsonism, amenable to gene therapy.
Abela L, Gianfrancesco L, Tagliatti E, Rossignoli G, Barwick K, Zourray C, Reid KM, Budinger D, Ng J, Counsell J, Simpson A, Pearson TS, Edvardson S, Elpeleg O, Brodsky FM, Lignani G, Barral S, Kurian MA. Abela L, et al. Brain. 2024 Jun 3;147(6):2023-2037. doi: 10.1093/brain/awae020. Brain. 2024. PMID: 38242634 Free PMC article. - Cross-linking mass spectrometry uncovers protein interactions and functional assemblies in synaptic vesicle membranes.
Wittig S, Ganzella M, Barth M, Kostmann S, Riedel D, Pérez-Lara Á, Jahn R, Schmidt C. Wittig S, et al. Nat Commun. 2021 Feb 8;12(1):858. doi: 10.1038/s41467-021-21102-w. Nat Commun. 2021. PMID: 33558502 Free PMC article. - Regulation of V-ATPase Activity and Organelle pH by Phosphatidylinositol Phosphate Lipids.
Banerjee S, Kane PM. Banerjee S, et al. Front Cell Dev Biol. 2020 Jun 23;8:510. doi: 10.3389/fcell.2020.00510. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32656214 Free PMC article. Review. - The emerging roles of vacuolar-type ATPase-dependent Lysosomal acidification in neurodegenerative diseases.
Song Q, Meng B, Xu H, Mao Z. Song Q, et al. Transl Neurodegener. 2020 May 11;9(1):17. doi: 10.1186/s40035-020-00196-0. Transl Neurodegener. 2020. PMID: 32393395 Free PMC article. Review. - Synaptojanin and Endophilin Mediate Neck Formation during Ultrafast Endocytosis.
Watanabe S, Mamer LE, Raychaudhuri S, Luvsanjav D, Eisen J, Trimbuch T, Söhl-Kielczynski B, Fenske P, Milosevic I, Rosenmund C, Jorgensen EM. Watanabe S, et al. Neuron. 2018 Jun 27;98(6):1184-1197.e6. doi: 10.1016/j.neuron.2018.06.005. Neuron. 2018. PMID: 29953872 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases