Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma - PubMed (original) (raw)
Clinical Trial
. 2018 Nov;24(11):1649-1654.
doi: 10.1038/s41591-018-0197-1. Epub 2018 Oct 8.
Sangeetha M Reddy 2, Hussein A Tawbi 1, Michael A Davies 1, Merrick I Ross 3, Isabella C Glitza 1, Janice N Cormier 3, Carol Lewis 4, Wen-Jen Hwu 1, Ehab Hanna 4, Adi Diab 1, Michael K Wong 1, Richard Royal 3, Neil Gross 4, Randal Weber 4, Stephen Y Lai 4, Richard Ehlers 3, Jorge Blando 5, Denái R Milton 6, Scott Woodman 1, Robin Kageyama 7, Daniel K Wells 7, Patrick Hwu 1, Sapna P Patel 1, Anthony Lucci 3, Amy Hessel 4, Jeffrey E Lee 3, Jeffrey Gershenwald 3, Lauren Simpson 1, Elizabeth M Burton 3, Liberty Posada 1, Lauren Haydu 3, Linghua Wang 8, Shaojun Zhang 8, Alexander J Lazar 9, Courtney W Hudgens 9, Vancheswaran Gopalakrishnan 3, Alexandre Reuben 3, Miles C Andrews 3, Christine N Spencer 8, Victor Prieto 9, Padmanee Sharma 5 10, James Allison 5, Michael T Tetzlaff 9 11, Jennifer A Wargo 12 13
Affiliations
- PMID: 30297909
- PMCID: PMC6481682
- DOI: 10.1038/s41591-018-0197-1
Clinical Trial
Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma
Rodabe N Amaria et al. Nat Med. 2018 Nov.
Erratum in
- Author Correction: Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma.
Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, Cormier JN, Lewis C, Hwu WJ, Hanna E, Diab A, Wong MK, Royal R, Gross N, Weber R, Lai SY, Ehlers R, Blando J, Milton DR, Woodman S, Kageyama R, Wells DK, Hwu P, Patel SP, Lucci A, Hessel A, Lee JE, Gershenwald J, Simpson L, Burton EM, Posada L, Haydu L, Wang L, Zhang S, Lazar AJ, Hudgens CW, Gopalakrishnan V, Reuben A, Andrews MC, Spencer CN, Prieto V, Sharma P, Allison J, Tetzlaff MT, Wargo JA. Amaria RN, et al. Nat Med. 2018 Dec;24(12):1941. doi: 10.1038/s41591-018-0251-z. Nat Med. 2018. PMID: 30361510 - Publisher Correction: Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma.
Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, Cormier JN, Lewis C, Hwu WJ, Hanna E, Diab A, Wong MK, Royal R, Gross N, Weber R, Lai SY, Ehlers R, Blando J, Milton DR, Woodman S, Kageyama R, Wells DK, Hwu P, Patel SP, Lucci A, Hessel A, Lee JE, Gershenwald J, Simpson L, Burton EM, Posada L, Haydu L, Wang L, Zhang S, Lazar AJ, Hudgens CW, Gopalakrishnan V, Reuben A, Andrews MC, Spencer CN, Prieto V, Sharma P, Allison J, Tetzlaff MT, Wargo JA. Amaria RN, et al. Nat Med. 2018 Dec;24(12):1942. doi: 10.1038/s41591-018-0252-y. Nat Med. 2018. PMID: 30361511
Abstract
Preclinical studies suggest that treatment with neoadjuvant immune checkpoint blockade is associated with enhanced survival and antigen-specific T cell responses compared with adjuvant treatment1; however, optimal regimens have not been defined. Here we report results from a randomized phase 2 study of neoadjuvant nivolumab versus combined ipilimumab with nivolumab in 23 patients with high-risk resectable melanoma ( NCT02519322 ). RECIST overall response rates (ORR), pathologic complete response rates (pCR), treatment-related adverse events (trAEs) and immune correlates of response were assessed. Treatment with combined ipilimumab and nivolumab yielded high response rates (RECIST ORR 73%, pCR 45%) but substantial toxicity (73% grade 3 trAEs), whereas treatment with nivolumab monotherapy yielded modest responses (ORR 25%, pCR 25%) and low toxicity (8% grade 3 trAEs). Immune correlates of response were identified, demonstrating higher lymphoid infiltrates in responders to both therapies and a more clonal and diverse T cell infiltrate in responders to nivolumab monotherapy. These results describe the feasibility of neoadjuvant immune checkpoint blockade in melanoma and emphasize the need for additional studies to optimize treatment regimens and to validate putative biomarkers.
Conflict of interest statement
Competing Interests: R.N.A. reports grants from Merck, Bristol-Myers Squibb and Array Biopharma, all outside the submitted work. S.M.R. received support from National Institutes of Health T32 Training Grant T32 CA 009666, outside the submitted work. H.A.T. reports personal fees from Novartis, grants from Merck and Celgene, and grants and personal fees from BMS and Genentech, all outside of the submitted work. M.A.D. reports personal fees from Novartis, BMS and Vaccinex, grants from AstraZeneca and Merck, and grants and personal fees from Roche/Genentech and Sanofi-Aventis, all outside the submitted work. W.-J.H. reports research grants from Merck, Bristol-Myers Squibb, MedImmune, GlaxoSmithKline and has served on an advisory board for Merck, all outside the submitted work. M.K.W. reports personal fees from Merck and EMD Serono, outside the submitted work. J.G. reports advisory board participation with Merck and Castle Biosciences. A.J.L. reports personal fees from BMS, personal fees from Novartis, personal fees from Genentech / Roche, personal fees and non-financial support from ArcherDX, personal fees and non-financial support from Beta-Cat, grants and non-financial support from Medimmune / AstraZeneca, personal fees from Merck, grants and non-financial support from Sanofi, grants, personal fees and non-financial support from Janssen, all outside the submitted work. V.G. reports US patent (PCT/US17/53,717), consultant fees from Microbiome DX, and honoraria from CAP18, outside of the submitted work. A.R. reports US patent (PCT/US17/53,717) and is supported by the Kimberley Clark Foundation Award for Scientific Achievement provided by MD Anderson’s Odyssey Fellowship Program. M.C.A. is supported by the National Health and Medical Research Council of Australia CJ Martin Early Career Fellowship (#1148680), and reports advisory board participation, travel support and honoraria from Merck Sharpe and Dohme. C.N.S. reports US patent (PCT/US17/53,717), outside of the submitted work. P.S. reports consultant or advisor fees from Bristol-Myers Squibb, GlaxoSmithKline, AstraZeneca, Amgen, Jounce, Kite Pharma, Neon, Evelo, EMD Serono, and Astellas, during the conduct of the study; stock from Jounce, Kite Pharma, Evelo, Constellation, Neon, outside the submitted work; and has a patent licensed to Jounce, outside the submitted work. M.T.T. reports personal fees from Myriad Genetics, Seattle Genetics and Novartis, all outside the submitted work. J.A.W. reports US patent (PCT/US17/53,717), has received compensation for speaker’s bureau and honoraria from Dava Oncology, Bristol-Myers Squibb and Illumina and has served on advisory committees for GlaxoSmithKline, Roche/Genentech, Novartis and AstraZeneca. All other authors declare no competing interests.
Figures
Figure 1.. Trial schema.
Patients with resectable clinical stage III or oligometastatic stage IV melanoma were stratified by stage and PD-L1 status and randomized in 1:1 ratio to neoadjuvant nivolumab 3 mg/kg for up to 4 doses (Arm A) or ipilimumab 3 mg/kg with nivolumab 1 mg/kg for up to 3 doses (Arm B), followed by surgical resection and then adjuvant nivolumab for 6 months. Primary endpoint of the trial was pathologic complete response (pCR) rate, defined as complete eradiation of tumor. Additional secondary endpoints included overall response rate by RECIST 1.1, survival outcomes, and immune correlates. Longitudinal tumor and blood samples were collected at baseline, prior to cycle 2, prior to cycle 3, and at the time of surgery followed by adjuvant blood collection every 3 months at the time of restaging.
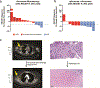
Figure 2.. Radiographic and pathologic responses to neoadjuvant nivolumab and combination ipilimumab with nivolumab.
a, Waterfall plots of overall response rates (ORR) by RECIST 1.1 at 8 weeks and surgical pathologic complete responses (pCR) for the patients treated with nivolumab monotherapy (n=12, 25% RECIST ORR and pCR rate). Two of the patients progressed beyond resectability and did not receive surgical resection. b, Combination ipilimumab with nivolumab (n=11) yielded improved outcomes compared to nivolumab, 73% (95% CI 39–94%, _p_=0.039) RECIST ORR and 45% (95% CI 17–77%, _p_=0.40) pCR rate using two-sided Fisher’s Exact Test. c, Only one patient on the trial who had a pCR also had a radiographic complete response, with the other pathologic responders having partial responses.
Figure 3.. Kaplan-Meier Estimates of Survival.
a,b,c, Kaplan-Meier estimates of progression-free survival (PFS), distant metastasis-free survival (DMFS), and overall survival (OS) by two-sided log-rank test are shown by treatment group. Patients were followed for a median of 15.0 months (range 5.8–22.6) in nivolumab (N) monotherapy arm (n=12) and 15.6 months (range 5.8–24.4) in the combination therapy (I+N) arm (n=11). Median survival endpoints have not been reached. Combination ipilimumab with nivolumab resulted in improved survival outcomes compared to nivolumab monotherapy, though this did not reach significance. For PFS, survival rates of 82% (95% CI 45–95%) at 17.2 months (mo) with I+N versus 58% (27–80%) at 22.6 mo with N, _p_=0.19. For DMFS, rates of 91% (51–99%) at 17.2 mo with I+N versus 67% (34–86%) at 22.6 mo with N, _p_=0.17. For OS, rates of 100% (100–100%) at 24.4 mo with I+N versus 76% (31–94%) at 22.6 mo with N, _p_=0.18.
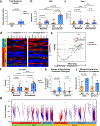
Figure 4.. Immune correlates of response to immune checkpoint blockade (ICB).
Longitudinal tumor biopsies were obtained at baseline and early on-treatment in patients on neoadjuvant ICB therapy. The tumor molecular and immune microenvironment was compared between non-responders (NR) and responders (R), with responders defined as patients achieving a complete or partial response by RECIST 1.1. a, Total mutational burden, defined as the sum of non-synonymous exonic mutations, is displayed (n=8 NR and 7 R). b,c, Quantification by immunohistochemistry of CD8 cell count density (n=11 NR and 10 R at baseline, n=11 NR and 9 R on-treatment) and PD-L1 H score (28–8 clone) (n=11 NR and 10 R at baseline, n=11 NR and 9 R on-treatment). d, Supervised hierarchical clustering summarizes expression of additional lymphoid markers (Granzyme B, CD4, FoxP3, CD20, and PD-1) using standard immunohistochemistry with blue indicating lower count density and red higher count density (or H score for PD-L1). e, Volcano plots of two-sided pairwise Mann-Whitney comparisons of multiplex immunohistochemistry marker expression between NR and R in baseline (green) and on-treatment (red) samples in the combined treatment arms (n=11 NR and 11 R at baseline, n=10 NR and 6 R on-treatment). Immune marker expression was assessed on CD45 positive cells and quantified as expression per area of assayed tissue. f, T cell clonality scores between NR and R at baseline (left, n=10 NR and 9 R) and on-treatment (right, n=10 NR and 9 R). g, Analysis of change in T cell repertoire was performed using differential abundance analysis. Clones that significantly increase (red) or decrease (blue) in frequency from baseline (closed circles) to on-treatment (open circles) are shown for whole clinical trial cohort. h,i, Change in T cell repertoire was assessed for number of significantly expanded clonotypes (n= 6 NR and 3 R for nivolumab treated patients, n=3 NR and 6 R for combination ICB treated patients) and Morisita Overlap index (n=6 NR ad 3 R for nivolumab treated patients, n=3 NR and 6 R for combination ICB treated patients). For a-c, f, h-I, Bar heights indicate median values, and interquartile ranges are presented in addition to individual data points. Comparisons were made using two-sided Mann-Whitney U tests.
Comment in
- New window of opportunity with ICIs in melanoma.
Killock D. Killock D. Nat Rev Clin Oncol. 2018 Dec;15(12):723. doi: 10.1038/s41571-018-0124-x. Nat Rev Clin Oncol. 2018. PMID: 30410079 No abstract available.
Similar articles
- Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma.
Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, Krijgsman O, van den Braber M, Philips D, Broeks A, van Thienen JV, Mallo HA, Adriaansz S, Ter Meulen S, Pronk LM, Grijpink-Ongering LG, Bruining A, Gittelman RM, Warren S, van Tinteren H, Peeper DS, Haanen JBAG, van Akkooi ACJ, Schumacher TN. Blank CU, et al. Nat Med. 2018 Nov;24(11):1655-1661. doi: 10.1038/s41591-018-0198-0. Epub 2018 Oct 8. Nat Med. 2018. PMID: 30297911 Clinical Trial. - Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial.
Rozeman EA, Menzies AM, van Akkooi ACJ, Adhikari C, Bierman C, van de Wiel BA, Scolyer RA, Krijgsman O, Sikorska K, Eriksson H, Broeks A, van Thienen JV, Guminski AD, Acosta AT, Ter Meulen S, Koenen AM, Bosch LJW, Shannon K, Pronk LM, Gonzalez M, Ch'ng S, Grijpink-Ongering LG, Stretch J, Heijmink S, van Tinteren H, Haanen JBAG, Nieweg OE, Klop WMC, Zuur CL, Saw RPM, van Houdt WJ, Peeper DS, Spillane AJ, Hansson J, Schumacher TN, Long GV, Blank CU. Rozeman EA, et al. Lancet Oncol. 2019 Jul;20(7):948-960. doi: 10.1016/S1470-2045(19)30151-2. Epub 2019 May 31. Lancet Oncol. 2019. PMID: 31160251 Clinical Trial. - Neoadjuvant Dual Checkpoint Inhibitors vs Anti-PD1 Therapy in High-Risk Resectable Melanoma: A Pooled Analysis.
Mangla A, Lee C, Mirsky MM, Wang M, Rothermel LD, Hoehn R, Bordeaux JS, Carroll BT, Theuner J, Li S, Fu P, Kirkwood JM. Mangla A, et al. JAMA Oncol. 2024 May 1;10(5):612-620. doi: 10.1001/jamaoncol.2023.7333. JAMA Oncol. 2024. PMID: 38546551 Free PMC article. - Neoadjuvant treatment for stage III and IV cutaneous melanoma.
Gorry C, McCullagh L, O'Donnell H, Barrett S, Schmitz S, Barry M, Curtin K, Beausang E, Barry R, Coyne I. Gorry C, et al. Cochrane Database Syst Rev. 2023 Jan 17;1(1):CD012974. doi: 10.1002/14651858.CD012974.pub2. Cochrane Database Syst Rev. 2023. PMID: 36648215 Free PMC article. Review. - Nivolumab: A Review in Advanced Melanoma.
Scott LJ. Scott LJ. Drugs. 2015 Aug;75(12):1413-24. doi: 10.1007/s40265-015-0442-6. Drugs. 2015. PMID: 26220912 Review.
Cited by
- Digital spatial profiling for pathologists.
Donati B, Manzotti G, Torricelli F, Ascione C, Valli R, Santandrea G, Ragazzi M, Zanetti E, Ciarrocchi A, Piana S. Donati B, et al. Virchows Arch. 2024 Nov 5. doi: 10.1007/s00428-024-03955-w. Online ahead of print. Virchows Arch. 2024. PMID: 39499318 - Neoadjuvant vidutolimod and nivolumab in high-risk resectable melanoma: A prospective phase II trial.
Davar D, Morrison RM, Dzutsev AK, Karunamurthy A, Chauvin JM, Amatore F, Deutsch JS, Das Neves RX, Rodrigues RR, McCulloch JA, Wang H, Hartman DJ, Badger JH, Fernandes MR, Bai Y, Sun J, Cole AM, Aggarwal P, Fang JR, Deitrick C, Bao R, Duvvuri U, Sridharan SS, Kim SW, A Choudry H, Holtzman MP, Pingpank JF, O'Toole JP, DeBlasio R, Jin Y, Ding Q, Gao W, Groetsch C, Pagliano O, Rose A, Urban C, Singh J, Divarkar P, Mauro D, Bobilev D, Wooldridge J, Krieg AM, Fury MG, Whiteaker JR, Zhao L, Paulovich AG, Najjar YG, Luke JJ, Kirkwood JM, Taube JM, Park HJ, Trinchieri G, Zarour HM. Davar D, et al. Cancer Cell. 2024 Nov 11;42(11):1898-1918.e12. doi: 10.1016/j.ccell.2024.10.007. Epub 2024 Oct 31. Cancer Cell. 2024. PMID: 39486411 Clinical Trial. - Immunoediting Dynamics in Glioblastoma: Implications for Immunotherapy Approaches.
Amin T, Hossain A, Jerin N, Mahmud I, Rahman MA, Rafiqul Islam SM, Islam SMBU. Amin T, et al. Cancer Control. 2024 Jan-Dec;31:10732748241290067. doi: 10.1177/10732748241290067. Cancer Control. 2024. PMID: 39353594 Free PMC article. Review. - Therapeutic Treatment Options for In-Transit Metastases from Melanoma.
Russano F, Rastrelli M, Dall'Olmo L, Del Fiore P, Gianesini C, Vecchiato A, Mazza M, Tropea S, Mocellin S. Russano F, et al. Cancers (Basel). 2024 Sep 3;16(17):3065. doi: 10.3390/cancers16173065. Cancers (Basel). 2024. PMID: 39272923 Free PMC article. Review. - Evidence for Radiation Therapy in Stage III Locoregionally Advanced Cutaneous Melanoma in the Post-Immunotherapy Era: A Literature Review.
Zhou J, Wuthrick E. Zhou J, et al. Cancers (Basel). 2024 Aug 30;16(17):3027. doi: 10.3390/cancers16173027. Cancers (Basel). 2024. PMID: 39272885 Free PMC article. Review.
References
- Liu J, et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer discovery 6, 1382–1399 (2016). - PubMed
- Eggermont AMM, Suciu S & Testori A Ipilimumab Adjuvant Therapy in Melanoma. The New England journal of medicine 376, 399 (2017). - PubMed
- Weber J, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. The New England journal of medicine 377, 1824–1835 (2017). - PubMed
- Eggermont AMM, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. The New England journal of medicine 378, 1789–1801 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical