TEAD4 overexpression promotes epithelial-mesenchymal transition and associates with aggressiveness and adverse prognosis in head neck squamous cell carcinoma - PubMed (original) (raw)
TEAD4 overexpression promotes epithelial-mesenchymal transition and associates with aggressiveness and adverse prognosis in head neck squamous cell carcinoma
Wei Zhang et al. Cancer Cell Int. 2018.
Erratum in
- Correction: TEAD4 overexpression promotes epithelial-mesenchymal transition and associates with aggressiveness and adverse prognosis in head neck squamous cell carcinoma.
Zhang W, Li J, Wu Y, Ge H, Song Y, Wang D, Yuan H, Jiang H, Wang Y, Cheng J. Zhang W, et al. Cancer Cell Int. 2024 Dec 19;24(1):417. doi: 10.1186/s12935-024-03577-x. Cancer Cell Int. 2024. PMID: 39702368 Free PMC article. No abstract available.
Abstract
Background: Deregulated Hippo signaling has been uncovered to be intricately involved in tumorigenesis. Transcriptional factor TEADs serve as key mediators of Hippo signaling and have been increasingly appreciated as putative oncogenes driving cancer initiation and progression. However, its expression pattern and oncogenic role of TEAD4 in head and neck squamous cell carcinoma (HNSCC) remain largely unexplored.
Methods: TEAD4 mRNA expression in HNSCC was determined by data mining and analyses from TCGA dataset and four independent cohorts with transcriptional profiling data publically available. The protein abundance of TEAD4 was measured by immunohistochemistry in 105 primary HNSCC samples and associations between its expression and clinicopathological parameters and patient survival were evaluated. The oncogenic roles of TEAD4 was further determined by 4-nitroquinoline 1-oxide (4NQO)-induced animal model, both knockdown/overexpression assay and TGF-β1-induced epithelia-mesenchymal transition (EMT) in vitro.
Results: Both mRNA and protein abundance of TEAD4 were significantly increased in HNSCC as compared to its non-tumor counterparts. Overexpression of TEAD4 significantly associated with high pathological grade, cervical node metastasis, advanced clinical stage and reduced overall and disease-free survival. In the 4NQO-induced HNSCC mouse model, increased TEAD4 immunostaining was found associated with disease progression. TEAD4 knockdown significantly inhibited cell proliferation, migration and invasion, and induced cell apoptosis in HNSCC cells, while its overexpression resulted in opposite effects and EMT. Moreover, TEAD4 was critically involved in TGF-β1-induced EMT in HNSCC cells.
Conclusions: Our findings reveal that TEAD4 serves as a novel prognostic biomarker and putative oncogene for HNSCC by promoting cell proliferation, migration and invasion, and EMT.
Keywords: EMT; Head and neck squamous cell carcinoma; Hippo signaling; Prognostic biomarker; TEAD4.
Figures
Fig. 1
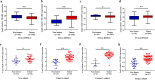
Overexpression of TEAD1-4 mRNA in HNSCC cohorts. The mRNA levels of TEAD1-4 (log2-transformed) were compared between HNSCC samples and normal counterparts in multiple patient cohorts. a–h The original data were retrieved from Oncomine database and TCGA and then plotted using GraphPad Prism 7 software. Y-axis represents the median intensity, 25th and 75th percentile data. *P < 0.05; **P < 0.01; ns, not significant; Student’s t test or Mann–Whitney U test as appropriate
Fig. 2
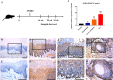
Immunohistochemical staining of TEAD4 in human HNSCC samples. A, B Representative negative staining of TEAD4 in normal oral epithelial; C, D representative low expression of TEAD4 in primary human HNSCC sample; E, F representative high expression of TEAD4 in primary human HNSCC sample. Nuclei are counterstained with hematoxylin. The areas marked by black box in the A, C, E images (upper panel) were shown in larger magnification as B, D, F images (lower panel), respectively. Scale bar: 100 μm
Fig. 3
High TEAD4 expression positively associates with reduced overall survival in HNSCC patients. Overall survival (a) and disease-free survival (b) analyses of patients stratified with high or low expression of TEAD4 were estimated by Kaplan–Meier method and compared with Log-rank test
Fig. 4
TEAD4 expression pattern during HNSCC tumorigenesis in 4NQO-induced animal model. A Experimental scheme of 4NQO-induced HNSCC animal model. B–I Immunohistochemical staining of TEAD4 in samples from diverse stages in 4NQO-induced animal model. Images in the upper panel (B, D, F, H) were representative staining of TEAD4 in normal, epithelial with hyperplasia, epithelial with severe dysplasia/carcinoma in situ and squamous cell carcinoma, respectively. Images in the lower panel (C, E, G, I) were magnified from the black box area in the B, D, F, H images in the upper panel, respectively. Scale bar: 100 μm. J The mRNA levels of TEAD4 during the 4NQO-induced HNSCC were measured by qRT-PCR in pre-stored samples (n = 5, 6 samples per group). *P < 0.05, **P < 0.01, ANOVA analyses
Fig. 5
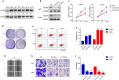
TEAD4 knockdown inhibits cell proliferation, migration and invasion, and triggers apoptosis in HNSCC cells. a Endogenous TEAD4 protein expression was measured in a panel of HNSCC cell lines as compared to normal oral epithelial (HOK). Representative images of western blot (WB) were shown from 3 independent experiments. b Endogenous TEAD4 was efficiently silenced by 2 siRNAs (siTEAD4–1, siTEAD4–2) in Cal27 and Fadu cells. Non-targeting siRNA was utilized as negative control (siNC). Representative images of WB are shown from 3 independent experiments. c Cell proliferation was remarkably suppressed when endogenous TEAD4 was silenced as measured by CCK-8 viability assay. d The potentials of colony formation were significantly inhibited in TEAD4-depleted cells (transfected with siTEAD4–1) as compared to control (siNC). e, f Increased percentages of cell undergoing apoptosis were evident following TEAD4 knockdown as assayed by Annexin V-PI staining. g–i The migration (g) and invasion (h) abilities were significantly reduced in siTEAD4-transfected cells in wound healing (12, 24 h after cell scratching) and transwell assays (12 h after cell seeding), respectively. Scale bar: 100 μm. Quantitive data of transwell assays were shown in i. Data shown here are mean ± SD from three independent experiments, *P < 0.05, **P < 0.01, ANOVA analyses with Tukey’s multiple comparisons test
Fig. 6
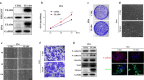
Ectopic TEAD4 overexpression induces EMT-like changes in HNSCC cells. a TEAD4 overexpression was confirmed by western blot in cellular lysates from 293T and HN6 cells infected with TEAD4 cDNA plasmid. Representative images of western blot (WB) were shown from 3 independent experiments. b Cell proliferation was remarkably promoted following TEAD4 overexpression by CCK-8 viability assay. c The potentials of colony formation were significantly promoted in TEAD4-overexpressed cells as compared to control. d Enforced TEAD4 overexpression resulted in EMT-like morphological changes from cobble-like to spindle-like appearance under phase contrast microscopy. e, f The cell motilities and invasion were remarkably enhanced after TEAD4 overexpression as gauged by wound healing (e) and transwell-invasion assay (f). Measurements of wound healing was performed at 6 and 12 h after cell scratching while measurements of transwell assays were done at 12 h after cell seeding. g The abundance of EMT markers E-cadherin, N-cadherin, Vimentin and Snail following TEAD4 overexpression were assayed by western blot (left panel). E-cadherin and vimentin expression was probed by immunofluorescent staining in TEAD4-overexpressed and control cells (right panel). Scale bar: 100 μm. Representative images are shown. Data showed here are mean ± SD from three independent experiments. **P < 0.01, Student-t test
Fig. 7
TEAD4 is required for TGF-β1-induced EMT in HNSCC cells. a The abundance of EMT-related markers E-cadherin, N-cadherin, vimentin and snail were measured by western blot (WB) in the Cal27 and Fadu cells following TEAD4 knockdown. b The mRNA and protein abundance of TEAD4 was measured by real-time RT-PCR and western blot when cells were treated with human recombinant TGF-β1 (10 ng/ml) at indicated time points. c The abundance of EMT-related markers E-cadherin, N-cadherin, vimentin and Snail were measured by immunofluorescence (c) and western blot assays (d) when Cal27 cells were treated siTEAD4 or in combination with TGF-β1 (10 ng/ml) for 48 h. e Cell migration was measured via wound healing assay when Cal27 cells were treated siTEAD4 or in combination with TGF-β1 (10 ng/ml) for 48 h. Scale bar: 100 μm. f Correlation between generic EMT score for 502 HNSCC samples from TCGA dataset and TEAD4 expression. Generic EMT score was calculated following the method of single-sample Gene Set Enrichment Analysis (ssGSEA) [36]. The correlation coefficient (R) and _P_-value were based on Pearson’s product-moment correlation analysis; R = 0.15, P = 0.0008. *P < 0.05, **P < 0.01, ANOVA analyses
Similar articles
- The Hippo effector TAZ promotes cancer stemness by transcriptional activation of SOX2 in head neck squamous cell carcinoma.
Li J, Li Z, Wu Y, Wang Y, Wang D, Zhang W, Yuan H, Ye J, Song X, Yang J, Jiang H, Cheng J. Li J, et al. Cell Death Dis. 2019 Aug 9;10(8):603. doi: 10.1038/s41419-019-1838-0. Cell Death Dis. 2019. PMID: 31399556 Free PMC article. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.
Bryant A, Hiu S, Kunonga PT, Gajjar K, Craig D, Vale L, Winter-Roach BA, Elattar A, Naik R. Bryant A, et al. Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review. - CCDC71L as a novel prognostic marker and immunotherapy target via lipid metabolism in head and neck squamous cell carcinoma.
Zhang Y, Tang H, Zi M, Zhang Z, Gao Q, Tian S. Zhang Y, et al. J Stomatol Oral Maxillofac Surg. 2024 Dec;125(6):101799. doi: 10.1016/j.jormas.2024.101799. Epub 2024 Feb 16. J Stomatol Oral Maxillofac Surg. 2024. PMID: 38367702 - Knockdown of Methylation-Related Gene MBD2 Blocks Cell Growth by Upregulating p21 Expression in Head and Neck Squamous Cell Carcinoma.
Cao T, Shen X, Pei F, Jiang T, Zhang J, Zhou H. Cao T, et al. Cancer Rep (Hoboken). 2024 Dec;7(12):e70080. doi: 10.1002/cnr2.70080. Cancer Rep (Hoboken). 2024. PMID: 39676597 Free PMC article.
Cited by
- Knockdown of HSP110 attenuates hypoxia-induced pulmonary hypertension in mice through suppression of YAP/TAZ-TEAD4 pathway.
Liu H, Zhang S, Liu Y, Ma J, Chen W, Yin T, Li T, Liang B, Tao L. Liu H, et al. Respir Res. 2022 Aug 19;23(1):209. doi: 10.1186/s12931-022-02124-4. Respir Res. 2022. PMID: 35986277 Free PMC article. - Regulation of TEAD Transcription Factors in Cancer Biology.
Huh HD, Kim DH, Jeong HS, Park HW. Huh HD, et al. Cells. 2019 Jun 17;8(6):600. doi: 10.3390/cells8060600. Cells. 2019. PMID: 31212916 Free PMC article. Review. - Discovery of a subtype-selective, covalent inhibitor against palmitoylation pocket of TEAD3.
Lu T, Li Y, Lu W, Spitters T, Fang X, Wang J, Cai S, Gao J, Zhou Y, Duan Z, Xiong H, Liu L, Li Q, Jiang H, Chen K, Zhou H, Lin H, Feng H, Zhou B, Antos CL, Luo C. Lu T, et al. Acta Pharm Sin B. 2021 Oct;11(10):3206-3219. doi: 10.1016/j.apsb.2021.04.015. Epub 2021 May 1. Acta Pharm Sin B. 2021. PMID: 34729310 Free PMC article. - Conservation of Epithelial-to-Mesenchymal Transition Process in Neural Crest Cells and Metastatic Cancer.
Zhang A, Aslam H, Sharma N, Warmflash A, Fakhouri WD. Zhang A, et al. Cells Tissues Organs. 2021;210(3):151-172. doi: 10.1159/000516466. Epub 2021 Jul 2. Cells Tissues Organs. 2021. PMID: 34218225 Free PMC article. - Epithelial-to-Mesenchymal Transition-Derived Heterogeneity in Head and Neck Squamous Cell Carcinomas.
Baumeister P, Zhou J, Canis M, Gires O. Baumeister P, et al. Cancers (Basel). 2021 Oct 26;13(21):5355. doi: 10.3390/cancers13215355. Cancers (Basel). 2021. PMID: 34771518 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials