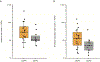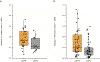Association of Tumor Microenvironment T-cell Repertoire and Mutational Load with Clinical Outcome after Sequential Checkpoint Blockade in Melanoma - PubMed (original) (raw)
Association of Tumor Microenvironment T-cell Repertoire and Mutational Load with Clinical Outcome after Sequential Checkpoint Blockade in Melanoma
Erik Yusko et al. Cancer Immunol Res. 2019 Mar.
Abstract
To understand prognostic factors for outcome between differentially sequenced nivolumab and ipilimumab in a randomized phase II trial, we measured T-cell infiltration and PD-L1 by IHC, T-cell repertoire metrics, and mutational load within the tumor. We used next-generation sequencing (NGS) and assessed the association of those parameters with response and overall survival. Immunosequencing of the T-cell receptor β-chain locus (TCRβ) from DNA of 91 pretreatment tumor samples and an additional 22 pairs of matched pre- and posttreatment samples from patients who received nivolumab followed by ipilimumab (nivo/ipi), or the reverse (ipi/nivo), was performed to measure T-cell clonality and fraction. Mutational and neoantigen load were also assessed by NGS in 82 of the 91 patients. Tumors were stained using IHC for PD-L1+ and CD8+ T cells. Pretreatment tumor TCR clonality and neoantigen load were marginally associated with best response with nivo/ipi (P = 0.04 and 0.05, respectively), but not with ipi/nivo. Amalgamated pretreatment mutational load and tumor T-cell fraction were significantly associated with best response with nivo/ipi (P = 0.002). Pretreatment PD-L1 staining intensity and CD8+ T-cell counts were correlated with T-cell fraction and clonality, but not mutational or neoantigen load. Patients with increased T-cell fraction posttreatment at week 13 had a 30-fold increased likelihood of survival (P = 0.002). Mutational and neoantigen load, and T-cell infiltrate within the tumor, were associated with outcome of sequential checkpoint inhibition using nivolumab then ipilimumab, but not when ipilimumab was administered before nivolumab.
©2019 American Association for Cancer Research.
Figures
Fig 1.
Mutational burden and neoantigen load versus clinical benefit in arm A (nivo/ipi). (A) Mutational burden (P = 0.06, n = 30). (B) Neoantigen load (P = 0.05, n = 30) for all patients calculated as described in Patients and Methods is shown on a log scale on the ordinate, and benefit defined as complete and partial response as well as stable disease (CR + PR) versus stable plus progressive disease (SD+PD) shown on the abscissa. The box plots show median (horizontal middle line), the interquartile range (IQR) (shaded box region), and the minimum and maximum (lines extended above and below the box). P values were obtained from a Mann–Whitney U Test.
Fig 2.
Pretreatment TIL clonality in tumor samples of arm A, and pretreatment T-cell fraction in tumor samples from both arms A and B, correlated with clinical benefit (P = 0.04 with N = 30 and P = 0.02 with N = 89, respectively). (A) Pretreatment T-cell clonality. (B) T-cell fraction calculated as described in Patients and Methods is shown on a linear scale on the ordinate with response (CR + PR) versus non-response (SD + PD) shown on the abscissa. P values are determined from a Mann–Whitney U Test.
Fig 3.
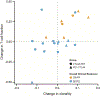
Mutation, T-cell fraction, and TIL clonality in pretreatment tumors predict clinical benefit in arm A. (A) ROC curves generated from an exhaustive leave-one-out cross-validation (LOOCV) procedure with a trivariate model (red line) with mutation, T-cell fraction, and TIL clonality compared to a univariate model (black line) employing PD-L1 expression. (B) Outcome of the multivariate logistic regression model, which included data from 34 patients. (C) Outcome of the univariate logistic regression model, including data from 30 patients. Two-dimensional projections of the TIL clonality and mutation burden in (D), T-cell fraction and mutational burden in (E), and T-cell fraction and mutation burden in (F). The color of the point or circle indicates clinical benefit, with closed circles indicating patients correctly classified and open circles indicating patients incorrectly classified in the LOOCV procedure.
Fig 4.
Increased on-treatment TIL clonality and T-cell fraction in tumor samples correlate with clinical benefit (P = 0.002, OR = 30). Changes in TIL clonality and T-cell fraction were determined by subtracting values determined at baseline from those determined in week 13 biopsies. P value was obtained from a Fisher’s exact test of CR/PR versus SD/PD comparing the top right quadrant to the remaining three quadrants.
Fig 5.
Overall survival curves for patients in arm A (30) with high T-cell fraction and mutational burden shown in orange, high T-cell fraction and low mutational burden in blue and low T-cell fraction and mutational burden in brown. High versus low was determined by the medians for each parameter.
Similar articles
- Nivolumab Plus Ipilimumab in Patients With Advanced Melanoma: Updated Survival, Response, and Safety Data in a Phase I Dose-Escalation Study.
Callahan MK, Kluger H, Postow MA, Segal NH, Lesokhin A, Atkins MB, Kirkwood JM, Krishnan S, Bhore R, Horak C, Wolchok JD, Sznol M. Callahan MK, et al. J Clin Oncol. 2018 Feb 1;36(4):391-398. doi: 10.1200/JCO.2017.72.2850. Epub 2017 Oct 17. J Clin Oncol. 2018. PMID: 29040030 Free PMC article. Clinical Trial. - Analyses of PD-L1 and Inflammatory Gene Expression Association with Efficacy of Nivolumab ± Ipilimumab in Gastric Cancer/Gastroesophageal Junction Cancer.
Lei M, Siemers NO, Pandya D, Chang H, Sanchez T, Harbison C, Szabo PM, Janjigian Y, Ott PA, Sharma P, Bendell J, Evans TRJ, de Braud F, Chau I, Boyd Z. Lei M, et al. Clin Cancer Res. 2021 Jul 15;27(14):3926-3935. doi: 10.1158/1078-0432.CCR-20-2790. Epub 2021 Mar 29. Clin Cancer Res. 2021. PMID: 33782030 - Ipilimumab and nivolumab combined with anthracycline-based chemotherapy in metastatic hormone receptor-positive breast cancer: a randomized phase 2b trial.
Andresen NK, Røssevold AH, Quaghebeur C, Gilje B, Boge B, Gombos A, Falk RS, Mathiesen RR, Julsrud L, Garred Ø, Russnes HG, Lereim RR, Chauhan SK, Lingjærde OC, Dunn C, Naume B, Kyte JA. Andresen NK, et al. J Immunother Cancer. 2024 Jan 19;12(1):e007990. doi: 10.1136/jitc-2023-007990. J Immunother Cancer. 2024. PMID: 38242720 Free PMC article. Clinical Trial. - Risks and benefits of reinduction ipilimumab/nivolumab in melanoma patients previously treated with ipilimumab/nivolumab.
Chapman PB, Jayaprakasam VS, Panageas KS, Callahan M, Postow MA, Shoushtari AN, Wolchok JD, Betof Warner A. Chapman PB, et al. J Immunother Cancer. 2021 Oct;9(10):e003395. doi: 10.1136/jitc-2021-003395. J Immunother Cancer. 2021. PMID: 34702752 Free PMC article. Review. - The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.
Mahoney KM, Freeman GJ, McDermott DF. Mahoney KM, et al. Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
Cited by
- Immune checkpoint therapy responders display early clonal expansion of tumor infiltrating lymphocytes.
Kidman J, Zemek RM, Sidhom JW, Correa D, Principe N, Sheikh F, Fear VS, Forbes CA, Chopra A, Boon L, Zaitouny A, de Jong E, Holt RA, Jones M, Millward MJ, Lassmann T, Forrest ARR, Nowak AK, Watson M, Lake RA, Lesterhuis WJ, Chee J. Kidman J, et al. Oncoimmunology. 2024 Apr 26;13(1):2345859. doi: 10.1080/2162402X.2024.2345859. eCollection 2024. Oncoimmunology. 2024. PMID: 38686178 Free PMC article. - Uveal melanoma immunogenomics predict immunotherapy resistance and susceptibility.
Leonard-Murali S, Bhaskarla C, Yadav GS, Maurya SK, Galiveti CR, Tobin JA, Kann RJ, Ashwat E, Murphy PS, Chakka AB, Soman V, Cantalupo PG, Zhuo X, Vyas G, Kozak DL, Kelly LM, Smith E, Chandran UR, Hsu YS, Kammula US. Leonard-Murali S, et al. Nat Commun. 2024 Apr 16;15(1):2863. doi: 10.1038/s41467-024-46906-4. Nat Commun. 2024. PMID: 38627362 Free PMC article. - Remodeling the tumor-immune microenvironment by anti-CTLA4 blockade enhanced subsequent anti-PD-1 efficacy in advanced nasopharyngeal carcinoma.
Ma Y, Zhou H, Luo F, Zhang Y, Zhu C, Li W, Huang Z, Zhao J, Xue J, Zhao Y, Fang W, Yang Y, Huang Y, Zhang L, Zhao H. Ma Y, et al. NPJ Precis Oncol. 2024 Mar 6;8(1):65. doi: 10.1038/s41698-024-00558-1. NPJ Precis Oncol. 2024. PMID: 38448521 Free PMC article. - Tumor mutational burden predictability in head and neck squamous cell carcinoma patients treated with immunotherapy: systematic review and meta-analysis.
Rodrigo JP, Sánchez-Canteli M, Otero-Rosales M, Martínez-Camblor P, Hermida-Prado F, García-Pedrero JM. Rodrigo JP, et al. J Transl Med. 2024 Feb 4;22(1):135. doi: 10.1186/s12967-024-04937-x. J Transl Med. 2024. PMID: 38311741 Free PMC article. Review. - Biomarkers and experimental models for cancer immunology investigation.
Xu H, Jia Z, Liu F, Li J, Huang Y, Jiang Y, Pu P, Shang T, Tang P, Zhou Y, Yang Y, Su J, Liu J. Xu H, et al. MedComm (2020). 2023 Dec 2;4(6):e437. doi: 10.1002/mco2.437. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38045830 Free PMC article. Review.
References
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L et al.: Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015. 372:320–330 - PubMed
- Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B et al.: Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. The Lancet Oncol 2015. 16:375–384 - PubMed
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L et al.: Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015. 372:2521–2532 - PubMed
- Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM et al. Durable Complete Response After Discontinuation of Pembrolizumab in Patients With Metastatic Melanoma. J Clin Oncol. 2018. 36(17):1668–1674 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials