Cell contact and Nf2/Merlin-dependent regulation of TEAD palmitoylation and activity - PubMed (original) (raw)
Cell contact and Nf2/Merlin-dependent regulation of TEAD palmitoylation and activity
Nam-Gyun Kim et al. Proc Natl Acad Sci U S A. 2019.
Abstract
The Hippo pathway is involved in regulating contact inhibition of proliferation and organ size control and responds to various physical and biochemical stimuli. It is a kinase cascade that negatively regulates the activity of cotranscription factors YAP and TAZ, which interact with DNA binding transcription factors including TEAD and activate the expression of target genes. In this study, we show that the palmitoylation of TEAD, which controls the activity and stability of TEAD proteins, is actively regulated by cell density independent of Lats, the key kinase of the Hippo pathway. The expression of fatty acid synthase and acetyl-CoA carboxylase involved in de novo biosynthesis of palmitate is reduced by cell density in an Nf2/Merlin-dependent manner. Depalmitoylation of TEAD is mediated by depalmitoylases including APT2 and ABHD17A. Palmitoylation-deficient TEAD4 mutant is unstable and degraded by proteasome through the activity of the E3 ubiquitin ligase CHIP. These findings show that TEAD activity is tightly controlled through the regulation of palmitoylation and stability via the orchestration of FASN, depalmitoylases, and E3 ubiquitin ligase in response to cell contact.
Keywords: Hippo signaling; TEAD; depalmitoylase; fatty acid synthase; palmitoylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Density-dependent regulation of YAP-TEAD activity. (A) The effects of cell density on YAP and TEAD protein expression. Parental (10A) and Lats1/2 KO (ΔLats) MCF-10A cells were grown at low (L) or high (H) cell densities. Cells were lysed, and cell lysates were separated on regular or 25 μM phos-tag conjugated SDS/PAGE and subjected to Western blot analysis with the indicated antibodies. The phosphorylation status of YAP was evaluated by using phos-tag analysis with anti-YAP or anti–phospho-YAP Ser127 antibodies. (B) Quantitative analysis of YAP-TEAD target genes’ mRNA expression. The expression of known YAP-TEAD target genes CTGF, Cyr61, and AREG in parental and Lats1/2 KO MCF-10A cells grown at low and high densities were analyzed using qPCR. (C) YAP-TEAD Reporter assay. 293T cells were transfected with control, Lats1/2, or YAP siRNA and HOP-flash reporter, a YAP/TAZ-TEAD activity reporter (41). Cells were harvested and reseeded at different densities, and luciferase activity was measured. Error bars represent SD. 293T cells were transfected with pcDNA3, p2xFlag-Lats1, or p2xFlag-Lats1-KD (K734M), a dominant negative mutant of Lats1, together with HOP-flash reporter and cultured at different cell densities before the luciferase assay. (D) The expression of exogenous TEAD4. Parental MCF-10A cells and MCF-10A cells stably expressing HA-tagged wild-type (WT) or YAP binding-deficient mutant (MT) TEAD4 were grown at different cell densities. E-cadherin and GAPDH were used as internal controls. (E) Regulation of exogenous TEAD4 protein expression by depletion of Hippo signaling components. MCF-10A cells stably expressing HA-tagged TEAD4 were transfected with indicated siRNAs and cultured at low or high cell densities.
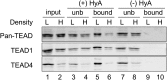
Fig. 2.
Density-dependent palmitoylation of TEAD. Acyl-RAC of TEAD in MCF-10A cells grown at low or high cell densities. The palmitoylation status of TEAD was analyzed using the Acyl-RAC technique. H, high cell density; HyA, hydroxylamine; L, low cell density; unb, unbound.
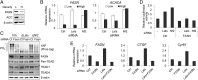
Fig. 3.
FASN-dependent regulation of YAP-TEAD activity. (A) Analysis of FASN and ACC expression. The expression of FASN and ACC proteins in MCF-10A at low and high cell densities was analyzed by Western blotting. H, high cell density; L, low cell density. (B) Quantitative analysis of FASN and ACACA mRNA expression. The expression of FASN and ACACA mRNAs in parental, and Lats1/2 or Nf2-depleted MCF-10A at low or high cell densities was analyzed using qPCR. (C) Biochemical effects of FASN depletion. Endogenous FASN was depleted by siRNA transfection in parental (10A), Lats1/2 (ΔLats), or Nf2 KO (ΔNf2) MCF-10A cells. Phosphorylation of YAP was monitored by phos-tag SDS/PAGE and Western blotting. (D) HOP-flash reporter. YAP-TEAD reporter assay was carried out in 293A cells transfected with control, Lats1/2, or Nf2 siRNAs in the presence/absence of FASN siRNA. (E) Quantitative analysis of YAP-TEAD target genes’ mRNA expression. The expressions of CTGF and Cyr61 mRNA in Lats1/2 and/or FASN-depleted MCF-10A cells were analyzed using qPCR.
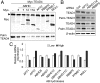
Fig. 4.
Identification of TEAD4 depalmitoylating enzymes. (A) Analysis of palmitoylation by Acyl-RAC analysis. Depalmitoylation of TEAD4 by the overexpression of serine hydrolases was determined by Acyl-RAC. (B) Increase of TEAD palmitoylation by the depletion of APT2. Endogenous serine hydrolases in MCF-10A cells were depleted by siRNA transfection. The palmitoylation status of TEAD1 and TEAD4 was determined by Acyl-RAC. (C) Quantitative analysis of serine hydrolases mRNA expression. The expression of serine hydrolases in MCF-10A cells at low or high densities was determined by qPCR.
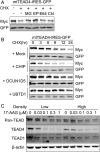
Fig. 5.
E3 ubiquitin ligase CHIP promotes the degradation of unpalmitoylated TEAD4. (A) Inhibition of palmitoylation-deficient TEAD4 degradation by proteasome inhibitors. 293A cells transfected with palmitoylation-deficient TEAD4-IRES-GFP (mtTEAD4-IRES-GFP) were treated with 10 μM CHX in the presence of MG (10 μM MG-132, proteasome inhibitor), EP (1 μM Epoxomicin, proteasome inhibitor), E64 (10 μM E-64, cysteine proteases inhibitor), or Chl (2.5 μM Chloroquine, autophagy inhibitor) for 9 h. The expression levels of mutant-TEAD4 and GFP were analyzed by Western blot. (B) Promotion of the degradation of unpalmitoylated TEAD4 by E3 ubiquitin ligase CHIP. 293A cells transfected with mtTEAD4-IRES-GFP and indicated E3-ubiquitin ligases. After 24 h of transfection, cells were treated with 10 μM CHX for the indicated time, followed by Western blot analysis. (C) Degradation of TEAD stimulated by the Hsp90 inhibitor 17-AAG. MCF-10A cells at low or high densities were treated with 17-AAG for 24 h at the indicated concentrations. MCF-10A cells at low or high densities were treated for 24 h with 17-AAG at the indicated concentrations. TEAD protein level was observed by Western blot.
Similar articles
- The tumor suppressor NF2 modulates TEAD4 stability and activity in Hippo signaling via direct interaction.
Wu M, Hu L, He L, Yuan L, Yang L, Zhao B, Zhang L, He X. Wu M, et al. J Biol Chem. 2024 May;300(5):107212. doi: 10.1016/j.jbc.2024.107212. Epub 2024 Mar 24. J Biol Chem. 2024. PMID: 38522513 Free PMC article. - Identification of resistance mechanisms to small-molecule inhibition of TEAD-regulated transcription.
Kulkarni A, Mohan V, Tang TT, Post L, Chan YC, Manning M, Thio N, Parker BL, Dawson MA, Rosenbluh J, Vissers JH, Harvey KF. Kulkarni A, et al. EMBO Rep. 2024 Sep;25(9):3944-3969. doi: 10.1038/s44319-024-00217-3. Epub 2024 Aug 5. EMBO Rep. 2024. PMID: 39103676 Free PMC article. - Allosteric Modulation of the YAP/TAZ-TEAD Interaction by Palmitoylation and Small-Molecule Inhibitors.
Mills KR, Misra J, Torabifard H. Mills KR, et al. J Phys Chem B. 2024 Apr 25;128(16):3795-3806. doi: 10.1021/acs.jpcb.3c07073. Epub 2024 Apr 12. J Phys Chem B. 2024. PMID: 38606592 - Regulation of the Hippo Pathway Transcription Factor TEAD.
Lin KC, Park HW, Guan KL. Lin KC, et al. Trends Biochem Sci. 2017 Nov;42(11):862-872. doi: 10.1016/j.tibs.2017.09.003. Epub 2017 Sep 27. Trends Biochem Sci. 2017. PMID: 28964625 Free PMC article. Review. - The Hippo Pathway and YAP/TAZ-TEAD Protein-Protein Interaction as Targets for Regenerative Medicine and Cancer Treatment.
Santucci M, Vignudelli T, Ferrari S, Mor M, Scalvini L, Bolognesi ML, Uliassi E, Costi MP. Santucci M, et al. J Med Chem. 2015 Jun 25;58(12):4857-73. doi: 10.1021/jm501615v. Epub 2015 Mar 11. J Med Chem. 2015. PMID: 25719868 Review.
Cited by
- Mitochondrial mechanotransduction through MIEF1 coordinates the nuclear response to forces.
Romani P, Benedetti G, Cusan M, Arboit M, Cirillo C, Wu X, Rouni G, Kostourou V, Aragona M, Giampietro C, Grumati P, Martello G, Dupont S. Romani P, et al. Nat Cell Biol. 2024 Dec;26(12):2046-2060. doi: 10.1038/s41556-024-01527-3. Epub 2024 Oct 21. Nat Cell Biol. 2024. PMID: 39433949 Free PMC article. - Hippo Signaling Pathway in Colorectal Cancer: Modulation by Various Signals and Therapeutic Potential.
Mohammadpour S, Torshizi Esfahani A, Sarpash S, Vakili F, Zafarjafarzadeh N, Mashaollahi A, Pardakhtchi A, Nazemalhosseini-Mojarad E. Mohammadpour S, et al. Anal Cell Pathol (Amst). 2024 Oct 11;2024:5767535. doi: 10.1155/2024/5767535. eCollection 2024. Anal Cell Pathol (Amst). 2024. PMID: 39431199 Free PMC article. Review. - [Advances in Targeted Therapy for Malignant Pleural Mesothelioma].
Fu F, Zhang Y, Shen H. Fu F, et al. Zhongguo Fei Ai Za Zhi. 2024 May 20;27(5):391-398. doi: 10.3779/j.issn.1009-3419.2024.102.18. Zhongguo Fei Ai Za Zhi. 2024. PMID: 38880927 Free PMC article. Review. Chinese. - NF2: An underestimated player in cancer metabolic reprogramming and tumor immunity.
Xu D, Yin S, Shu Y. Xu D, et al. NPJ Precis Oncol. 2024 Jun 15;8(1):133. doi: 10.1038/s41698-024-00627-5. NPJ Precis Oncol. 2024. PMID: 38879686 Free PMC article. Review. - The RNF214-TEAD-YAP signaling axis promotes hepatocellular carcinoma progression via TEAD ubiquitylation.
Lin M, Zheng X, Yan J, Huang F, Chen Y, Ding R, Wan J, Zhang L, Wang C, Pan J, Cao X, Fu K, Lou Y, Feng XH, Ji J, Zhao B, Lan F, Shen L, He X, Qiu Y, Jin J. Lin M, et al. Nat Commun. 2024 Jun 11;15(1):4995. doi: 10.1038/s41467-024-49045-y. Nat Commun. 2024. PMID: 38862474 Free PMC article.
References
- Chan EH, et al. (2005) The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24:2076–2086. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous