Motor dysfunction and neurodegeneration in a C9orf72 mouse line expressing poly-PR - PubMed (original) (raw)
Motor dysfunction and neurodegeneration in a C9orf72 mouse line expressing poly-PR
Zongbing Hao et al. Nat Commun. 2019.
Abstract
A GGGGCC hexanucleotide repeat expansion in intron 1 of chromosome 9 open reading frame 72 (C9ORF72) gene is the most common genetic cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia. Repeat-associated non-ATG translation of dipeptide repeat proteins (DPRs) contributes to the neuropathological features of c9FTD/ALS. Among the five DPRs, arginine-rich poly-PR are reported to be the most toxic. Here, we generate a transgenic mouse line that expresses poly-PR (GFP-PR28) specifically in neurons. GFP-PR28 homozygous mice show decreased survival time, while the heterozygous mice show motor imbalance, decreased brain weight, loss of Purkinje cells and lower motor neurons, and inflammation in the cerebellum and spinal cord. Transcriptional analysis shows that in the cerebellum, GFP-PR28 heterozygous mice show differential expression of genes related to synaptic transmission. Our findings show that GFP-PR28 transgenic mice partly model neuropathological features of c9FTD/ALS, and show a role for poly-PR in neurodegeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures
Fig. 1
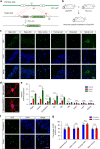
Distribution of GFP-PR28 in heterozygous mice. a Diagram of the construct containing GFP-PR 28 flox/flox in Rosa26 site. b Breeding scheme for producing GFP-PR 28 flox/+ ;Thy1-Cre + heterozygous mice driven by Thy1 promoter for neuronal expressions. c Representative images showing distribution of poly-PR aggregates in different brain regions of GFP-PR28 heterozygous mice at 2 months of age. GFP (green), Hoechst (blue). Scale bar represents 50 μm. d Representative images showing the distribution of GFP-PR28 in GFAP or Iba1-positive glia in the motor cortex of 6-month-old GFP-PR28 heterozygous mice. GFP (green), Hoechst (Blue), GFAP/Iba1 (Red). Scale bar represents 10 μm. e Relative mRNA levels of GFP in different brain regions and tissues of 20-day-old control, GFP-PR28 heterozygous and homozygous mice. Hippocampus (Hippo), spinal cord (SC), brainstem (BS), olfactory bulb (OB). One-way ANOVA, Bonferroni post hoc test; n = 3–4 mice per group. f Representative images showing the distribution of poly-PR aggregates in the cerebellum and motor cortex of GFP-PR28 heterozygous mice at 12 months of age. GFP (green), Hoechst (Blue). Scale bar represents 50 μm. g Percentage of cells containing GFP-PR28 in different brain regions of GFP-PR28 heterozygous mice at 2 and 12 months of age. Two-way ANOVA, Bonferroni post hoc test; n = 5 mice per group. All data were displayed as mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n.s. not significant
Fig. 2
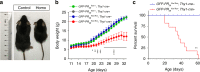
GFP-PR28 homozygous mice display reduced body weight and decreased survival. a Representative image showing the body size of the control and homozygous mice at 20 days of age. b Body weight curves of control, heterozygous, and the homozygous mice from postnatal day 11 to 33. Two-way ANOVA, Bonferroni post hoc test; n = 4, 6, 6, 6 mice. c Kaplan–Meier survival curve of the control and homozygous mice. Gehan–Breslow–Wilcoxon test, n = 12, 11 mice. Control: GFP-PR 28 flox/flox ;Thy1-Cre-, Homo: GFP-PR 28 flox/flox ;Thy1-Cre + (c). All data are displayed as mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Fig. 3
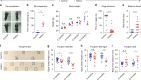
GFP-PR28 heterozygous mice show motor deficits. a Representative images of male control and GFP-PR28 heterozygous mice at 2 months of age in tail-suspension test. b Quantification of the clasping time of mice in (a) during 2 -min test. Mann–Whitney test, n = 8, 6 mice. c Body weight of male control and GFP-PR28 heterozygous mice at 2, 4, 6, and 12 months old. Two-way ANOVA, Bonferroni post hoc test; n = 5, 7 mice. d Quantification of time keeping on the edges of cages of male control and GFP-PR28 heterozygous mice at 6 months of age. Mann–Whitney test, n = 19, 18 mice. e The numbers of hind limb foot slips on the balance beam test of male control and GFP-PR28 heterozygous mice at 6 months of age. Mann–Whitney test, n = 19, 18 mice. f Representative images of male control and GFP-PR28 heterozygous mice at 12 months of age in footprint test. Fore paws (red), hind paws (blue). Black squares indicate localization of hind paws. g Back stride length (left) of 6-, 12-month-old male control and GFP-PR28 heterozygous mice measured in footprint test. Two-way ANOVA, Bonferroni post hoc test; 6 months, n = 22, 14 mice; 12 months, n = 16, 8 mice. h Back stride length (right) of 6-, 12-month-old male control and GFP-PR28 heterozygous mice measured in footprint test. Two-way ANOVA, Bonferroni post hoc test; 6 months, n = 22, 14 mice; 12 months, n = 16, 8 mice. i Back-front distance measured in footprint test of male control and GFP-PR28 heterozygous mice at 6, 12 months of age. Two-way ANOVA, Bonferroni post hoc test; 6 months, n = 22, 14 mice; 12 months, n = 16, 8 mice. All data are displayed as mean ± s.e.m. *P < 0.05, ***P < 0.001, ****P < 0.0001, n.s. not significant
Fig. 4
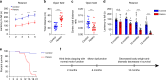
GFP-PR28 heterozygous mice show progressive motor deficits and anxiety-like behaviors. a Latencies to fall from the accelerated rotating beams of male control and GFP-PR28 heterozygous mice at 6 months of age. Two-way ANOVA, Bonferroni post hoc test; n = 18, 18 mice. b, c Open-field test showing the ratio of time in the center area and total ambulatory distance of male control and GFP-PR28 heterozygous mice at 6 months of age. Two-tailed t test, n = 20, 18 mice. d Latencies to fall from the accelerated rotating beams of control and GFP-PR28 heterozygous mice at 3, 6, 10, and 12–16 months of age. Two-way ANOVA, Bonferroni post hoc test; 3 months, n = 17, 9 mice; 6 months, n = 12, 11 mice; 10 months, n = 17, 11 mice; 12–16 months, n = 17, 10 mice. e Kaplan–Meier survival curve of control and heterozygous mice. Gehan–Breslow–Wilcoxon test, n = 32, 24 mice. f Diagram of the disease progression of GFP-PR28 heterozygous mice. All data are displayed as mean ± s.e.m. *P < 0.05, ***P < 0.001, ****P < 0.0001, n.s. not significant
Fig. 5
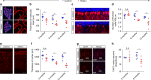
Expression of GFP-PR28 causes motor-related neurodegeneration. a Representative images showing the size of the cerebellum and the thickness of the molecular layer of control and GFP-PR28 heterozygous mice at 6 months of age. Neu-N (red), Hoechst (blue). White squares indicates enlarged area. Green lines indicate the thickness of the molecular layer. Scale bar represents 100 μm. b Quantification of the thickness of molecular layer of control and GFP-PR28 heterozygous mice at 2, 6, and 12 months of age. Two months, n = 5, 5 mice; 6 months, n = 5, 5 mice; 12 months, n = 4, 6 mice. c Representative images showing the numbers of cerebellar Purkinje cells of 6-month-old control and GFP-PR28 heterozygous mice. Calbindin (red), Hoechst (blue). Scale bar represents 50 μm. d Quantification of the numbers of calbindin positive Purkinje cells of control and GFP-PR28 heterozygous mice at 2, 6 and 12 months of age. Two months, n = 5, 5 mice; 6 months, n = 5, 5 mice; 12 months, n = 4, 5 mice. e Representative images showing the thickness of motor cortex of control and GFP-PR28 heterozygous mice at 6 months of age. Scale bar represents 100 μm. f Quantification of the thickness of the motor cortex of control and GFP-PR28 heterozygous mice at 2, 6, and 12 months of age. Two months, n = 5, 5 mice; 6 months, n = 5, 4 mice; 12 months, n = 4, 5 mice. g Representative images showing the numbers of ChAT-positive motor neurons in the lumbar spinal cord of 6-month-old control and GFP-PR28 heterozygous mice. GM (gray matter), WM (white matter). Scale bar represents 100 μm. h Quantification of the numbers of ChAT-positive motor neurons of control and GFP-PR28 heterozygous mice at 2 and 6 months of age. Two months, n = 3, 3 mice; 6 months, n = 5, 5 mice. All data aredisplayed as mean ± s.e.m. Two-way ANOVA, Bonferroni post hoc test; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n.s. not significant
Fig. 6
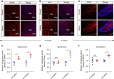
Gliosis in the lumbar spinal cord and cerebellum of GFP-PR28 heterozygous mice. a Representative images showing the numbers of GFAP-positive astrocytes in the lumbar spinal cord of 6-month-old control and GFP-PR28 heterozygous mice. GM (gray matter), WM (white matter). White squares are enlarged views of corresponding images. GFAP (red), Hoechst (blue). Scale bar represents 100 μm. b Representative images showing the numbers of Iba1-positive microglia in the lumbar spinal cord of 6-month-old control and GFP-PR28 heterozygous mice. GM (gray matter), WM (white matter). White squares are enlarged views of corresponding images. Iba1 (red), Hoechst (blue). Scale bar represents 100 μm. c Relative integrated optical density of GFAP in the lumbar spinal cord of control and heterozygous mice at 2 and 6 months of age. Two-way ANOVA, Bonferroni post hoc test; 2 months, n = 3, 3 mice; 6 months, n = 3, 3 mice. d Relative integrated optical density of Iba1 in the lumbar spinal cord of control and heterozygous mice at 2 and 6 months of age. Two-way ANOVA, Bonferroni post hoc test; 2 months, n = 3, 3 mice; 6 months, n = 3, 3 mice. e Representative images showing the numbers of GFAP-positive astrocytes in the cerebellum of 6-month-old control and GFP-PR28 heterozygous mice. GFAP (red), Hoechst (blue). Scale bar represents 100 μm. f Relative integrated optical density of GFAP in the cerebellum of control and heterozygous mice at 2 and 6 months of age. Two-way ANOVA, Bonferroni post hoc test; 2 months, n = 5, 4 mice; 6 months, n = 4, 5 mice. All data are displayed as mean ± s.e.m. *P < 0.05, **P < 0.01, n.s. not significant
Fig. 7
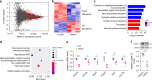
GFP-PR28 expression is associated with synaptic transmission-related genes dysregulation. a MA-plot of differentially expressed genes in the cerebellum of 5-month-old control and GFP-PR28 heterozygous mice. Red blots indicate significant changes, n = 3, 3 mice. b Hierarchical clustering of differentially expressed genes in the cerebellum of 5-month-old control and GFP-PR28 heterozygous mice. c Gene ontology (GO) biological processes analyses of upregulated and downregulated genes in (b). d The Reactome pathway analyses of top five enriched terms of downregulated genes in (b). Gene numbers indicate genes that are enriched in this pathway. Rich factor indicates the ratio of enriched genes to total genes in this pathway. e Relative mRNA expression of six genes (Camk4, Grin2a, Kcnj9, Rims3, Syt2, and Unc13a) in association with synaptic transmission in the cerebellum of 5-month-old control and GFP-PR28 heterozygous mice. Two-tailed t test, n = 4, 4 mice. f Relative expressing levels of CaMK IV in the cerebellum of 6-month-old control and GFP-PR28 heterozygous mice, determined by western blotting assay. Two-tailed t test, CaMK IV, n = 5, 5 mice. All data are displayed as mean ± s.e.m. *P < 0.05, **P < 0.01, ****P < 0.0001
Similar articles
- Sense-encoded poly-GR dipeptide repeat proteins correlate to neurodegeneration and uniquely co-localize with TDP-43 in dendrites of repeat-expanded C9orf72 amyotrophic lateral sclerosis.
Saberi S, Stauffer JE, Jiang J, Garcia SD, Taylor AE, Schulte D, Ohkubo T, Schloffman CL, Maldonado M, Baughn M, Rodriguez MJ, Pizzo D, Cleveland D, Ravits J. Saberi S, et al. Acta Neuropathol. 2018 Mar;135(3):459-474. doi: 10.1007/s00401-017-1793-8. Epub 2017 Dec 1. Acta Neuropathol. 2018. PMID: 29196813 Free PMC article. - Reactivation of nonsense-mediated mRNA decay protects against C9orf72 dipeptide-repeat neurotoxicity.
Xu W, Bao P, Jiang X, Wang H, Qin M, Wang R, Wang T, Yang Y, Lorenzini I, Liao L, Sattler R, Xu J. Xu W, et al. Brain. 2019 May 1;142(5):1349-1364. doi: 10.1093/brain/awz070. Brain. 2019. PMID: 30938419 Free PMC article. - The DNA damage response (DDR) is induced by the C9orf72 repeat expansion in amyotrophic lateral sclerosis.
Farg MA, Konopka A, Soo KY, Ito D, Atkin JD. Farg MA, et al. Hum Mol Genet. 2017 Aug 1;26(15):2882-2896. doi: 10.1093/hmg/ddx170. Hum Mol Genet. 2017. PMID: 28481984 - Molecular Mechanisms of Neurodegeneration Related to C9orf72 Hexanucleotide Repeat Expansion.
Babić Leko M, Župunski V, Kirincich J, Smilović D, Hortobágyi T, Hof PR, Šimić G. Babić Leko M, et al. Behav Neurol. 2019 Jan 15;2019:2909168. doi: 10.1155/2019/2909168. eCollection 2019. Behav Neurol. 2019. PMID: 30774737 Free PMC article. Review. - Arginine-rich dipeptide-repeat proteins as phase disruptors in C9-ALS/FTD.
Odeh HM, Shorter J. Odeh HM, et al. Emerg Top Life Sci. 2020 Dec 11;4(3):293-305. doi: 10.1042/ETLS20190167. Emerg Top Life Sci. 2020. PMID: 32639008 Free PMC article. Review.
Cited by
- _C9orf72_-Derived Proline:Arginine Poly-Dipeptides Modulate Cytoskeleton and Mechanical Stress Response.
Shiota T, Nagata R, Kikuchi S, Nanaura H, Matsubayashi M, Nakanishi M, Kobashigawa S, Isozumi N, Kiriyama T, Nagayama K, Sugie K, Yamashiro Y, Mori E. Shiota T, et al. Front Cell Dev Biol. 2022 Mar 23;10:750829. doi: 10.3389/fcell.2022.750829. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35399536 Free PMC article. - Microglia and Astrocytes in Amyotrophic Lateral Sclerosis: Disease-Associated States, Pathological Roles, and Therapeutic Potential.
You J, Youssef MMM, Santos JR, Lee J, Park J. You J, et al. Biology (Basel). 2023 Oct 3;12(10):1307. doi: 10.3390/biology12101307. Biology (Basel). 2023. PMID: 37887017 Free PMC article. Review. - Glucose hypometabolism prompts RAN translation and exacerbates C9orf72-related ALS/FTD phenotypes.
Nelson AT, Cicardi ME, Markandaiah SS, Han JY, Philp NJ, Welebob E, Haeusler AR, Pasinelli P, Manfredi G, Kawamata H, Trotti D. Nelson AT, et al. EMBO Rep. 2024 May;25(5):2479-2510. doi: 10.1038/s44319-024-00140-7. Epub 2024 Apr 29. EMBO Rep. 2024. PMID: 38684907 Free PMC article. - A robust evaluation of TDP-43, poly GP, cellular pathology and behavior in an AAV-C9ORF72 (G4C2)66 mouse model.
Thompson EG, Spead O, Akerman SC, Curcio C, Zaepfel BL, Kent ER, Philips T, Vijayakumar BG, Zacco A, Zhou W, Nagappan G, Rothstein JD. Thompson EG, et al. Acta Neuropathol Commun. 2024 Dec 26;12(1):203. doi: 10.1186/s40478-024-01911-y. Acta Neuropathol Commun. 2024. PMID: 39722074 Free PMC article. - C9ORF72: What It Is, What It Does, and Why It Matters.
Smeyers J, Banchi EG, Latouche M. Smeyers J, et al. Front Cell Neurosci. 2021 May 5;15:661447. doi: 10.3389/fncel.2021.661447. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34025358 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous