The Role of Post-Translational Modifications in the Phase Transitions of Intrinsically Disordered Proteins - PubMed (original) (raw)
Review
The Role of Post-Translational Modifications in the Phase Transitions of Intrinsically Disordered Proteins
Izzy Owen et al. Int J Mol Sci. 2019.
Abstract
Advances in genomics and proteomics have revealed eukaryotic proteomes to be highly abundant in intrinsically disordered proteins that are susceptible to diverse post-translational modifications. Intrinsically disordered regions are critical to the liquid-liquid phase separation that facilitates specialized cellular functions. Here, we discuss how post-translational modifications of intrinsically disordered protein segments can regulate the molecular condensation of macromolecules into functional phase-separated complexes.
Keywords: intrinsically disordered regions; liquid–liquid phase separation; membraneless organelles; post-translational modifications.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
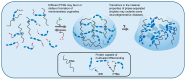
Figure 1
Liquid–liquid phase separation (LLPS) of biopolymers, such as proteins and RNA, is a mechanism by which cells organize their contents into specific functional structures called membraneless organelles (MLOs). Post-translational modifications (PTMs) of intrinsically disordered proteins can influence LLPS and thus regulate the formation and dissolution of MLOs. The figure depicts different patterns of PTMs favoring dispersed or condensed states. Changes in the material properties of liquid-phase separated granules are hypothesized to cause some neurodegenerative diseases. According to this hypothesis, droplets lose their liquid (reversible) properties and adopt more rigid (less reversible) internal structures, which may be glass-like, or in some cases, may have solid amyloid-like structures. These irreversible phase states may have gain-of-function toxicity to neurons.
Similar articles
- Intrinsically Disordered Proteome of Human Membrane-Less Organelles.
Darling AL, Liu Y, Oldfield CJ, Uversky VN. Darling AL, et al. Proteomics. 2018 Mar;18(5-6):e1700193. doi: 10.1002/pmic.201700193. Epub 2017 Dec 21. Proteomics. 2018. PMID: 29068531 Review. - Single-molecule fluorescence studies of intrinsically disordered proteins and liquid phase separation.
Nasir I, Onuchic PL, Labra SR, Deniz AA. Nasir I, et al. Biochim Biophys Acta Proteins Proteom. 2019 Oct;1867(10):980-987. doi: 10.1016/j.bbapap.2019.04.007. Epub 2019 May 2. Biochim Biophys Acta Proteins Proteom. 2019. PMID: 31054969 Free PMC article. Review. - Membraneless organelles: P granules in Caenorhabditis elegans.
Marnik EA, Updike DL. Marnik EA, et al. Traffic. 2019 Jun;20(6):373-379. doi: 10.1111/tra.12644. Epub 2019 Apr 11. Traffic. 2019. PMID: 30924287 Free PMC article. Review. - Disordered domains in chromatin-binding proteins.
Watson M, Stott K. Watson M, et al. Essays Biochem. 2019 Apr 23;63(1):147-156. doi: 10.1042/EBC20180068. Print 2019 Apr 23. Essays Biochem. 2019. PMID: 30940742 Review. - Modulation of Intrinsically Disordered Protein Function by Post-translational Modifications.
Bah A, Forman-Kay JD. Bah A, et al. J Biol Chem. 2016 Mar 25;291(13):6696-705. doi: 10.1074/jbc.R115.695056. Epub 2016 Feb 5. J Biol Chem. 2016. PMID: 26851279 Free PMC article. Review.
Cited by
- Balanced Force Field ff03CMAP Improving the Dynamics Conformation Sampling of Phosphorylation Site.
Zhong B, Song G, Chen HF. Zhong B, et al. Int J Mol Sci. 2022 Sep 25;23(19):11285. doi: 10.3390/ijms231911285. Int J Mol Sci. 2022. PMID: 36232586 Free PMC article. - Host casein kinase 1-mediated phosphorylation modulates phase separation of a rhabdovirus phosphoprotein and virus infection.
Fang XD, Gao Q, Zang Y, Qiao JH, Gao DM, Xu WY, Wang Y, Li D, Wang XB. Fang XD, et al. Elife. 2022 Feb 22;11:e74884. doi: 10.7554/eLife.74884. Elife. 2022. PMID: 35191833 Free PMC article. - Liquid-Liquid Phase Separation: Unraveling the Enigma of Biomolecular Condensates in Microbial Cells.
Gao Z, Zhang W, Chang R, Zhang S, Yang G, Zhao G. Gao Z, et al. Front Microbiol. 2021 Oct 25;12:751880. doi: 10.3389/fmicb.2021.751880. eCollection 2021. Front Microbiol. 2021. PMID: 34759902 Free PMC article. Review. - From Prions to Stress Granules: Defining the Compositional Features of Prion-Like Domains That Promote Different Types of Assemblies.
Fomicheva A, Ross ED. Fomicheva A, et al. Int J Mol Sci. 2021 Jan 27;22(3):1251. doi: 10.3390/ijms22031251. Int J Mol Sci. 2021. PMID: 33513942 Free PMC article. Review. - Biomolecular condensates as arbiters of biochemical reactions inside the nucleus.
Laflamme G, Mekhail K. Laflamme G, et al. Commun Biol. 2020 Dec 15;3(1):773. doi: 10.1038/s42003-020-01517-9. Commun Biol. 2020. PMID: 33319830 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources