Critical Role of Cytosolic DNA and Its Sensing Adaptor STING in Aortic Degeneration, Dissection, and Rupture - PubMed (original) (raw)
. 2020 Jan 7;141(1):42-66.
doi: 10.1161/CIRCULATIONAHA.119.041460. Epub 2019 Dec 30.
Yidan Wang 1 2, Lin Zhang 1 2, Pingping Ren 1 2, Chen Zhang 1 2, Yanming Li 1 2, Alon R Azares 3, Michelle Zhang 1, Jiao Guo 1 2, Ketan B Ghaghada 4, Zbigniew A Starosolski 4, Kimal Rajapakshe 5, Cristian Coarfa 6, Yumei Li 7, Rui Chen 8 9 7, Keigi Fujiwara 10, Jun-Ichi Abe 10, Joseph S Coselli 11 1 2, Dianna M Milewicz 12, Scott A LeMaire 11 1 2, Ying H Shen 11 1 2
Affiliations
- PMID: 31887080
- PMCID: PMC6939474
- DOI: 10.1161/CIRCULATIONAHA.119.041460
Critical Role of Cytosolic DNA and Its Sensing Adaptor STING in Aortic Degeneration, Dissection, and Rupture
Wei Luo et al. Circulation. 2020.
Abstract
Background: Sporadic aortic aneurysm and dissection (AAD), caused by progressive aortic smooth muscle cell (SMC) loss and extracellular matrix degradation, is a highly lethal condition. Identifying mechanisms that drive aortic degeneration is a crucial step in developing an effective pharmacologic treatment to prevent disease progression. Recent evidence has indicated that cytosolic DNA and abnormal activation of the cytosolic DNA sensing adaptor STING (stimulator of interferon genes) play a critical role in vascular inflammation and destruction. Here, we examined the involvement of this mechanism in aortic degeneration and sporadic AAD formation.
Methods: The presence of cytosolic DNA in aortic cells and activation of the STING pathway were examined in aortic tissues from patients with sporadic ascending thoracic AAD. The role of STING in AAD development was evaluated in _Sting_-deficient (Sting gt/gt) mice in a sporadic AAD model induced by challenging mice with a combination of a high-fat diet and angiotensin II. We also examined the direct effects of STING on SMC death and macrophage activation in vitro.
Results: In human sporadic AAD tissues, we observed the presence of cytosolic DNA in SMCs and macrophages and significant activation of the STING pathway. In the sporadic AAD model, Sting gt/gt mice showed significant reductions in challenge-induced aortic enlargement, dissection, and rupture in both the thoracic and abdominal aortic regions. Single-cell transcriptome analysis revealed that aortic challenge in wild-type mice induced the DNA damage response, the inflammatory response, dedifferentiation and cell death in SMCs, and matrix metalloproteinase expression in macrophages. These changes were attenuated in challenged Sting gt/gt mice. Mechanistically, nuclear and mitochondrial DNA damage in SMCs and the subsequent leak of DNA to the cytosol activated STING signaling, which induced cell death through apoptosis and necroptosis. In addition, DNA from damaged SMCs was engulfed by macrophages in which it activated STING and its target interferon regulatory factor 3, which directly induced matrix metalloproteinase-9 expression. We also found that pharmacologically inhibiting STING activation partially prevented AAD development.
Conclusions: Our findings indicate that the presence of cytosolic DNA and subsequent activation of cytosolic DNA sensing adaptor STING signaling represent a key mechanism in aortic degeneration and that targeting STING may prevent sporadic AAD development.
Keywords: STING; aortic aneurysm; aortic dissection; cell death; cytosolic DNA; matrix metalloproteinases.
Conflict of interest statement
Disclosures
The authors have no potential conflicts of interest to disclose.
Figures
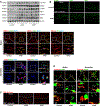
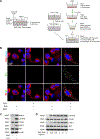
Figure 1. Presence of cytosolic DNA and activation of the STING signaling pathway in human sporadic ascending thoracic aortic aneurysm and dissection (ATAAD) tissues.
Aortic tissues from patients with ascending thoracic aortic aneurysm (ATAA) (n=10), patients with acute ascending thoracic aortic dissection (ATAD) (n=10), and organ donors (control) (n=8) were analyzed. A, Representative western blot data showing that the STING pathway was activated in the aortic wall of ATAAD patients. B, Immunostaining showing that the levels of STING, TBK1, and IRF3 were increased in diseased aortas, particularly in smooth muscle cells (SM22-α) and macrophages (CD68). Insets show a higher magnification view. C, Representative images of dihydroethidium (DHE) staining showing increased reactive oxygen species production in the aortic wall of ATAAD patients. D, Representative immunostaining of DNA and mitochondria (Tomm20) showing cytosolic DNA (not located in nuclei or mitochondria) in smooth muscle cells of the aortic media and in macrophages of the adventitia in ATAD tissues. Insets show a higher magnification view. Asterisk indicates nuclear DNA (nDNA). Arrowhead indicates mitochondrial DNA (mtDNA). Arrow indicates cytosolic DNA (ctDNA).
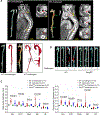
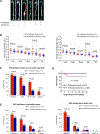
Figure 2. Reduced incidence of aortic aneurysm and dissection (AAD) and preserved aortic structure and contractile ability in challenged _Sting_-deficient (Sting gt/gt) mice.
Wild-type (WT) mice and _Sting_gt/gt mice were unchallenged or challenged with a high-fat diet for 8 weeks and angiotensin II infusion (2000 ng/min/kg) during the last 4 weeks. A, Representative computed tomography angiography (CTA) images of aortas from challenged WT mice showing the presence of AAD in the thoracic and suprarenal aortic segments. B, Representative images of excised aortas showing less aortic damage in challenged Sting gt/gt mice than in challenged WT mice. C, Mean aortic diameters of various aortic segments were smaller in challenged Sting gt/gt mice than in challenged WT mice. The measurements were based on the excised aortas (as shown in B). Asc, ascending; Desc, descending; SR, suprarenal; IR, infrarenal. D, The overall incidences of AAD, severe AAD, and rupture were significantly lower in challenged Sting gt/gt mice than in challenged WT mice. E, Kaplan-Meier survival analysis showing improved survival in challenged Sting gt/gt mice compared with challenged WT mice during the 4 weeks of angiotensin II infusion. F, The incidence of AAD in different aortic segments was significantly lower in challenged Sting gt/gt mice than in challenged WT mice. G, The lower overall AAD incidence in challenged Sting gt/gt mice was similar in male and female mice. H, Representative hematoxylin and eosin (H&E) staining and Verhoeff–van Gieson elastin staining (elastin) of ascending aortic sections showing preserved aortic structure in _Sting_gt/gt mice compared with challenged WT mice. The quantification of aortic elastic fiber fragmentation showing less aortic destruction in challenged _Sting_gt/gt mice than in challenged WT mice. I, Wire myograph analysis of ascending thoracic aortic rings showing a significantly reduced contractile response to phenylephrine in challenged WT mice compared with unchallenged WT mice. Challenged Sting gt/gt mice exhibited partial preservation of contractile ability. Two-way ANOVA with the Bonferroni post-hoc test was used for pairwise comparisons in (C) and (H). The Fisher exact test was used for (D), (F), and (G). Multi-way analysis of variance with the Holm-Šídák test was used for pairwise comparisons in (I). ***P<0.001. Data are presented as the mean ± standard error of the mean.
Figure 2. Reduced incidence of aortic aneurysm and dissection (AAD) and preserved aortic structure and contractile ability in challenged _Sting_-deficient (Sting gt/gt) mice.
Wild-type (WT) mice and _Sting_gt/gt mice were unchallenged or challenged with a high-fat diet for 8 weeks and angiotensin II infusion (2000 ng/min/kg) during the last 4 weeks. A, Representative computed tomography angiography (CTA) images of aortas from challenged WT mice showing the presence of AAD in the thoracic and suprarenal aortic segments. B, Representative images of excised aortas showing less aortic damage in challenged Sting gt/gt mice than in challenged WT mice. C, Mean aortic diameters of various aortic segments were smaller in challenged Sting gt/gt mice than in challenged WT mice. The measurements were based on the excised aortas (as shown in B). Asc, ascending; Desc, descending; SR, suprarenal; IR, infrarenal. D, The overall incidences of AAD, severe AAD, and rupture were significantly lower in challenged Sting gt/gt mice than in challenged WT mice. E, Kaplan-Meier survival analysis showing improved survival in challenged Sting gt/gt mice compared with challenged WT mice during the 4 weeks of angiotensin II infusion. F, The incidence of AAD in different aortic segments was significantly lower in challenged Sting gt/gt mice than in challenged WT mice. G, The lower overall AAD incidence in challenged Sting gt/gt mice was similar in male and female mice. H, Representative hematoxylin and eosin (H&E) staining and Verhoeff–van Gieson elastin staining (elastin) of ascending aortic sections showing preserved aortic structure in _Sting_gt/gt mice compared with challenged WT mice. The quantification of aortic elastic fiber fragmentation showing less aortic destruction in challenged _Sting_gt/gt mice than in challenged WT mice. I, Wire myograph analysis of ascending thoracic aortic rings showing a significantly reduced contractile response to phenylephrine in challenged WT mice compared with unchallenged WT mice. Challenged Sting gt/gt mice exhibited partial preservation of contractile ability. Two-way ANOVA with the Bonferroni post-hoc test was used for pairwise comparisons in (C) and (H). The Fisher exact test was used for (D), (F), and (G). Multi-way analysis of variance with the Holm-Šídák test was used for pairwise comparisons in (I). ***P<0.001. Data are presented as the mean ± standard error of the mean.
Figure 2. Reduced incidence of aortic aneurysm and dissection (AAD) and preserved aortic structure and contractile ability in challenged _Sting_-deficient (Sting gt/gt) mice.
Wild-type (WT) mice and _Sting_gt/gt mice were unchallenged or challenged with a high-fat diet for 8 weeks and angiotensin II infusion (2000 ng/min/kg) during the last 4 weeks. A, Representative computed tomography angiography (CTA) images of aortas from challenged WT mice showing the presence of AAD in the thoracic and suprarenal aortic segments. B, Representative images of excised aortas showing less aortic damage in challenged Sting gt/gt mice than in challenged WT mice. C, Mean aortic diameters of various aortic segments were smaller in challenged Sting gt/gt mice than in challenged WT mice. The measurements were based on the excised aortas (as shown in B). Asc, ascending; Desc, descending; SR, suprarenal; IR, infrarenal. D, The overall incidences of AAD, severe AAD, and rupture were significantly lower in challenged Sting gt/gt mice than in challenged WT mice. E, Kaplan-Meier survival analysis showing improved survival in challenged Sting gt/gt mice compared with challenged WT mice during the 4 weeks of angiotensin II infusion. F, The incidence of AAD in different aortic segments was significantly lower in challenged Sting gt/gt mice than in challenged WT mice. G, The lower overall AAD incidence in challenged Sting gt/gt mice was similar in male and female mice. H, Representative hematoxylin and eosin (H&E) staining and Verhoeff–van Gieson elastin staining (elastin) of ascending aortic sections showing preserved aortic structure in _Sting_gt/gt mice compared with challenged WT mice. The quantification of aortic elastic fiber fragmentation showing less aortic destruction in challenged _Sting_gt/gt mice than in challenged WT mice. I, Wire myograph analysis of ascending thoracic aortic rings showing a significantly reduced contractile response to phenylephrine in challenged WT mice compared with unchallenged WT mice. Challenged Sting gt/gt mice exhibited partial preservation of contractile ability. Two-way ANOVA with the Bonferroni post-hoc test was used for pairwise comparisons in (C) and (H). The Fisher exact test was used for (D), (F), and (G). Multi-way analysis of variance with the Holm-Šídák test was used for pairwise comparisons in (I). ***P<0.001. Data are presented as the mean ± standard error of the mean.
Figure 3. Prevention of the aortic challenge–induced expression of genes involved in smooth muscle cell (SMC) death and inflammation in Sting gt/gt mice.
A-C, Bulk RNA-sequencing (RNA-seq) analysis was performed in ascending aortas from wild-type (WT) mice and Sting gt/gt mice that were unchallenged or challenged with a high-fat diet (HFD) for 5 weeks and angiotensin II (AngII; 2000 ng/min/kg) infusion during the last week. For each group, 5 aortas were pooled as 1 sample, and duplicate samples were tested. A, Heatmap showing different gene expression patterns among groups. B, Gene ontology (GO) analysis showing that aortic challenge induced the expression of genes involved in several biologic processes (left) and that this induction was prevented in Sting gt/gt mice (right). C, Heatmap showing that Sting deficiency prevented the challenge-induced upregulation of genes involved in the STING pathway, cell death pathways (apoptosis, necroptosis, and pyroptosis), and the inflammatory response D-I, Single-cell transcriptome analysis was performed in ascending aortas from WT mice and _Sting_gt/gt mice that were unchallenged or challenged with a HFD for 5 weeks AngII (2000 ng/min/kg) infusion during the last week. For each group, single cell suspensions from 3 aortas were pooled as one sample. VSMC, vascular smooth muscle cell. D, t-Stochastic neighbor embedding (t-SNE) plots from the ascending aortas of WT mice showing 12 different cell clusters, including 6 clusters of SMCs and 2 clusters of macrophages. E, Sting expression was projected onto t-SNE plots of challenged WT mice showing the high expression of Sting in SMCs, macrophages, and fibroblasts (left). Quantification showing that aortic challenge increased Sting gene expression in SMC cluster 5 and SMC cluster 6 (right). F, GO enrichment analysis of _Sting_-positive SMCs (cluster 5) showing that aortic challenge induced the expression of several genes with roles in different biologic processes (up) but that this was prevented in challenged _Sting_- deficient mice (down). G, Heatmap of _Sting_-positive SMCs (cluster 5) showing that Sting deficiency prevented the challenge-induced expression of genes in inflammation and cell death (apoptosis, necroptosis, and pyroptosis). H, GO enrichment analysis of _Sting_-positive macrophages (cluster 11) showing that Sting deficiency prevented the aortic challenge-induced expression of several genes. I, Heatmap of _Sting_-positive macrophages (cluster 11) showing that Sting deficiency prevented the challenge-induced upregulation of genes involved in inflammation.
Figure 3. Prevention of the aortic challenge–induced expression of genes involved in smooth muscle cell (SMC) death and inflammation in Sting gt/gt mice.
A-C, Bulk RNA-sequencing (RNA-seq) analysis was performed in ascending aortas from wild-type (WT) mice and Sting gt/gt mice that were unchallenged or challenged with a high-fat diet (HFD) for 5 weeks and angiotensin II (AngII; 2000 ng/min/kg) infusion during the last week. For each group, 5 aortas were pooled as 1 sample, and duplicate samples were tested. A, Heatmap showing different gene expression patterns among groups. B, Gene ontology (GO) analysis showing that aortic challenge induced the expression of genes involved in several biologic processes (left) and that this induction was prevented in Sting gt/gt mice (right). C, Heatmap showing that Sting deficiency prevented the challenge-induced upregulation of genes involved in the STING pathway, cell death pathways (apoptosis, necroptosis, and pyroptosis), and the inflammatory response D-I, Single-cell transcriptome analysis was performed in ascending aortas from WT mice and _Sting_gt/gt mice that were unchallenged or challenged with a HFD for 5 weeks AngII (2000 ng/min/kg) infusion during the last week. For each group, single cell suspensions from 3 aortas were pooled as one sample. VSMC, vascular smooth muscle cell. D, t-Stochastic neighbor embedding (t-SNE) plots from the ascending aortas of WT mice showing 12 different cell clusters, including 6 clusters of SMCs and 2 clusters of macrophages. E, Sting expression was projected onto t-SNE plots of challenged WT mice showing the high expression of Sting in SMCs, macrophages, and fibroblasts (left). Quantification showing that aortic challenge increased Sting gene expression in SMC cluster 5 and SMC cluster 6 (right). F, GO enrichment analysis of _Sting_-positive SMCs (cluster 5) showing that aortic challenge induced the expression of several genes with roles in different biologic processes (up) but that this was prevented in challenged _Sting_- deficient mice (down). G, Heatmap of _Sting_-positive SMCs (cluster 5) showing that Sting deficiency prevented the challenge-induced expression of genes in inflammation and cell death (apoptosis, necroptosis, and pyroptosis). H, GO enrichment analysis of _Sting_-positive macrophages (cluster 11) showing that Sting deficiency prevented the aortic challenge-induced expression of several genes. I, Heatmap of _Sting_-positive macrophages (cluster 11) showing that Sting deficiency prevented the challenge-induced upregulation of genes involved in inflammation.
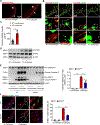
Figure 4. Prevention of challenge-induced smooth muscle cell (SMC) death and macrophage MMP-9 production in _Sting_-deficient (Sting gt/gt) mice.
Analyses were performed by using ascending aortas from wild-type (WT) mice and Sting gt/gt mice that were unchallenged or challenged with a high-fat diet for 8 weeks and angiotensin II infusion (2000 ng/min/kg) during the last 4 weeks. A, Representative images of dihydroethidium (DHE) staining showing increased reactive oxygen species production in the aortic wall (M, media; A, adventitia) of challenged wild-type (WT) mice. B, Representative immunostaining of DNA and mitochondria (Tomm20) showing cytosolic DNA in SMCs of the aortic media and in macrophages of the adventitia in challenged WT mice (M: media; A: adventitia). Insets show a higher-magnification view. Asterisk indicates nuclear DNA. Arrowhead indicates mitochondrial DNA. Arrow indicates cytosolic DNA. C, Bar graph showing that the quantity of cytosolic double-stranded DNA (dsDNA) was increased in aortic cells of the ascending aorta of challenged WT mice compared with unchallenged WT mice (n=5 per group). D, Western blot analysis showing that aortic challenge in WT mice induced a marked increase in the levels of phosphorylated STING in the ascending aorta (n=3 per group). E, Western blot analysis and quantification data showing that the cleavage of caspase-3 and PARP-1 was reduced in ascending aortas from challenged Sting gt/gt mice compared with those from challenged WT mice (n=6 per group). F, Representative terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-stained images and quantification data showing that the number of apoptotic aortic SMCs was reduced in challenged Sting gt/gt mice compared with challenged WT mice (n=4 per group) (M: media; A: adventitia). G, Western blot analysis and quantification data showing that the phosphorylation of RIP3 (p-RIP3) and MLKL (p-MLKL) in the ascending aorta was reduced in challenged Sting gt/gt mice compared with challenged WT mice (n=6 per group). H, Representative immunofluorescence staining and quantification data showing that the levels of p-RIP3 and p-MLKL in aortic SMCs (SM22-α) were reduced in challenged Sting gt/gt mice compared with challenged WT mice (n=4 per group) (M: media; A: adventitia). I, Western blot analysis and quantification showing that MMP-9 expression in the ascending aorta was reduced in challenged Sting gt/gt mice compared with challenged WT mice (n=6 per group). J, Representative immunofluorescence staining showing that MMP-9 expression in macrophages (CD68) was decreased in aortas from challenged Sting gt/gt mice compared with those from challenged WT mice (M: media; A: adventitia). K, In situ zymography results showing that MMP activity in the aorta was decreased in challenged Sting gt/gt mice compared with challenged WT mice. Insets show a higher magnification view. An unpaired two-tailed _t_-test was used in (C). Two-way ANOVA with the Bonferroni post-hoc test was used for pairwise comparisons in (E) through (I). *P<0.05, **P<0.01, ***P<0.001. Data are presented as the mean ± standard error of the mean.
Figure 4. Prevention of challenge-induced smooth muscle cell (SMC) death and macrophage MMP-9 production in _Sting_-deficient (Sting gt/gt) mice.
Analyses were performed by using ascending aortas from wild-type (WT) mice and Sting gt/gt mice that were unchallenged or challenged with a high-fat diet for 8 weeks and angiotensin II infusion (2000 ng/min/kg) during the last 4 weeks. A, Representative images of dihydroethidium (DHE) staining showing increased reactive oxygen species production in the aortic wall (M, media; A, adventitia) of challenged wild-type (WT) mice. B, Representative immunostaining of DNA and mitochondria (Tomm20) showing cytosolic DNA in SMCs of the aortic media and in macrophages of the adventitia in challenged WT mice (M: media; A: adventitia). Insets show a higher-magnification view. Asterisk indicates nuclear DNA. Arrowhead indicates mitochondrial DNA. Arrow indicates cytosolic DNA. C, Bar graph showing that the quantity of cytosolic double-stranded DNA (dsDNA) was increased in aortic cells of the ascending aorta of challenged WT mice compared with unchallenged WT mice (n=5 per group). D, Western blot analysis showing that aortic challenge in WT mice induced a marked increase in the levels of phosphorylated STING in the ascending aorta (n=3 per group). E, Western blot analysis and quantification data showing that the cleavage of caspase-3 and PARP-1 was reduced in ascending aortas from challenged Sting gt/gt mice compared with those from challenged WT mice (n=6 per group). F, Representative terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-stained images and quantification data showing that the number of apoptotic aortic SMCs was reduced in challenged Sting gt/gt mice compared with challenged WT mice (n=4 per group) (M: media; A: adventitia). G, Western blot analysis and quantification data showing that the phosphorylation of RIP3 (p-RIP3) and MLKL (p-MLKL) in the ascending aorta was reduced in challenged Sting gt/gt mice compared with challenged WT mice (n=6 per group). H, Representative immunofluorescence staining and quantification data showing that the levels of p-RIP3 and p-MLKL in aortic SMCs (SM22-α) were reduced in challenged Sting gt/gt mice compared with challenged WT mice (n=4 per group) (M: media; A: adventitia). I, Western blot analysis and quantification showing that MMP-9 expression in the ascending aorta was reduced in challenged Sting gt/gt mice compared with challenged WT mice (n=6 per group). J, Representative immunofluorescence staining showing that MMP-9 expression in macrophages (CD68) was decreased in aortas from challenged Sting gt/gt mice compared with those from challenged WT mice (M: media; A: adventitia). K, In situ zymography results showing that MMP activity in the aorta was decreased in challenged Sting gt/gt mice compared with challenged WT mice. Insets show a higher magnification view. An unpaired two-tailed _t_-test was used in (C). Two-way ANOVA with the Bonferroni post-hoc test was used for pairwise comparisons in (E) through (I). *P<0.05, **P<0.01, ***P<0.001. Data are presented as the mean ± standard error of the mean.
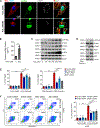
Figure 5. Critical role of the STING-TBK1-IRF3 pathway in aortic smooth muscle cell (SMC) injury.
A, Representative immunostaining of DNA, mitochondria (MitoTracker), and lysosomes (LAMP1) showing mitochondrial DNA (mtDNA; double-stranded DNA [dsDNA] that colocalized with MitoTracker), nuclear DNA (dsDNA in the nuclei), and cytosolic DNA (dsDNA that did not colocalize with either mitochondria, nuclei, or lysosomes) in cultured SMCs (n=5 biologic repeats). H2O2 treatment increased the amount of cytosolic DNA in SMCs. Insets show a higher magnification view. B, Bar graph showing that the concentration of cytosolic dsDNA was increased in H2O2-treated SMCs compared with control SMCs (n=4 biologic repeats). C, Western blot analysis showing that H2O2 increased the phosphorylation of STING, TBK1, and IRF3 in SMCs in a dose-dependent manner. D, Western blot results showing that transfection with exogenous SMC DNA from H2O2-treated SMCs activated the STING pathway in SMCs. E, Flow cytometry analyses showing that silencing STING, TBK1, or IRF3 with siRNA partially prevented cell death induced by H2O2 or exogenous SMC DNA (n=4 biologic repeats). F, Flow cytometry analysis showing that silencing STING, TBK1, or IRF3 with siRNA prevented necroptotic cell death induced by H2O2 or exogenous SMC DNA (n=4 or 5 biologic repeats). PI, propidium iodide. G, Western blot analysis showing that H2O2 or exogenous SMC DNA induced the expression and phosphorylation of RIP3 and MLKL in a dose-dependent manner in SMCs pretreated with zVAD. H, Western blot results showing that silencing STING, TBK1, or IRF3 with siRNA prevented the H2O2− or exogenous SMC DNA–induced activation and phosphorylation of RIP3 and MLKL in SMCs pretreated with zVAD. I, Western blot results showing that the overexpression of STING or TBK1 increased the activation and phosphorylation of RIP3 and MLKL in SMCs pretreated with zVAD. An unpaired two-tailed _t_-test was used in (B). Two-way ANOVA with Bonferroni’s post-hoc test for pairwise comparisons was used in (E) and (F). ns indicates not significant. **P<0.01, ***P<0.001. Data are presented as the mean ± standard error of the mean.
Figure 5. Critical role of the STING-TBK1-IRF3 pathway in aortic smooth muscle cell (SMC) injury.
A, Representative immunostaining of DNA, mitochondria (MitoTracker), and lysosomes (LAMP1) showing mitochondrial DNA (mtDNA; double-stranded DNA [dsDNA] that colocalized with MitoTracker), nuclear DNA (dsDNA in the nuclei), and cytosolic DNA (dsDNA that did not colocalize with either mitochondria, nuclei, or lysosomes) in cultured SMCs (n=5 biologic repeats). H2O2 treatment increased the amount of cytosolic DNA in SMCs. Insets show a higher magnification view. B, Bar graph showing that the concentration of cytosolic dsDNA was increased in H2O2-treated SMCs compared with control SMCs (n=4 biologic repeats). C, Western blot analysis showing that H2O2 increased the phosphorylation of STING, TBK1, and IRF3 in SMCs in a dose-dependent manner. D, Western blot results showing that transfection with exogenous SMC DNA from H2O2-treated SMCs activated the STING pathway in SMCs. E, Flow cytometry analyses showing that silencing STING, TBK1, or IRF3 with siRNA partially prevented cell death induced by H2O2 or exogenous SMC DNA (n=4 biologic repeats). F, Flow cytometry analysis showing that silencing STING, TBK1, or IRF3 with siRNA prevented necroptotic cell death induced by H2O2 or exogenous SMC DNA (n=4 or 5 biologic repeats). PI, propidium iodide. G, Western blot analysis showing that H2O2 or exogenous SMC DNA induced the expression and phosphorylation of RIP3 and MLKL in a dose-dependent manner in SMCs pretreated with zVAD. H, Western blot results showing that silencing STING, TBK1, or IRF3 with siRNA prevented the H2O2− or exogenous SMC DNA–induced activation and phosphorylation of RIP3 and MLKL in SMCs pretreated with zVAD. I, Western blot results showing that the overexpression of STING or TBK1 increased the activation and phosphorylation of RIP3 and MLKL in SMCs pretreated with zVAD. An unpaired two-tailed _t_-test was used in (B). Two-way ANOVA with Bonferroni’s post-hoc test for pairwise comparisons was used in (E) and (F). ns indicates not significant. **P<0.01, ***P<0.001. Data are presented as the mean ± standard error of the mean.
Figure 6. DNA from aortic smooth muscle cells (SMCs) induced MMP-9 production in macrophages through the STING pathway.
A, Illustration of the transwell co-culture system. The upper schematic shows that the transwell comprised a 24-well culture insert with a 0.4-μm pore size in the upper part and a 24-well plate in the lower part. The lower schematic shows the experimental procedure. PMA, phorbol 12-myristate 13-acetate. B, Immunofluorescence staining showing the presence of DNA from Edu-labeled H2O2-treated SMCs in the cytosol of macrophages (CD68) after using a transwell co-culture system. Cells are outlined with white dots. Nuclei are outlined with yellow dots. C, Western blot showing that MMP-9 expression and the phosphorylation of STING and IRF3 were increased in macrophages that were co-cultured with H2O2-treated SMCs. D-G, Macrophages were transfected with DNA isolated from H2O2-treated SMCs. SMC-derived DNA increased the expression and phosphorylation of STING and IRF3. (D), SMC-derived DNA also increased levels of MMP-9 protein (E), MMP9 mRNA (F), and MMP-9 activity (G) (n=5 biologic repeats). H-K, Macrophages were transfected with STING or IRF3 plasmid DNA or transfected with STING or IRF3 siRNA, followed by stimulation with SMC DNA. MMP-9 protein levels (n=4 biologic repeats) (H), MMP9 mRNA levels (n=3 biologic repeats) (I), and MMP-9 activity (J) were enhanced by the overexpression of STING or IRF3 or reduced by the knockdown of STING or IRF3. K, The results of a chromatin immunoprecipitation assay showing that IRF3 bound to the MMP9 promoter in unstressed macrophages. This binding was increased in cells treated with SMC DNA and was abolished by inhibiting STING expression with STING siRNA (n=4 biologic repeats). An unpaired, two-tailed _t_-test was used in (F) and (I). Two-way ANOVA with the Bonferroni post-hoc test for pairwise comparisons was used in (I). **P<0.01, ***P<0.001. Data are presented as the mean ± standard error of the mean.
Figure 6. DNA from aortic smooth muscle cells (SMCs) induced MMP-9 production in macrophages through the STING pathway.
A, Illustration of the transwell co-culture system. The upper schematic shows that the transwell comprised a 24-well culture insert with a 0.4-μm pore size in the upper part and a 24-well plate in the lower part. The lower schematic shows the experimental procedure. PMA, phorbol 12-myristate 13-acetate. B, Immunofluorescence staining showing the presence of DNA from Edu-labeled H2O2-treated SMCs in the cytosol of macrophages (CD68) after using a transwell co-culture system. Cells are outlined with white dots. Nuclei are outlined with yellow dots. C, Western blot showing that MMP-9 expression and the phosphorylation of STING and IRF3 were increased in macrophages that were co-cultured with H2O2-treated SMCs. D-G, Macrophages were transfected with DNA isolated from H2O2-treated SMCs. SMC-derived DNA increased the expression and phosphorylation of STING and IRF3. (D), SMC-derived DNA also increased levels of MMP-9 protein (E), MMP9 mRNA (F), and MMP-9 activity (G) (n=5 biologic repeats). H-K, Macrophages were transfected with STING or IRF3 plasmid DNA or transfected with STING or IRF3 siRNA, followed by stimulation with SMC DNA. MMP-9 protein levels (n=4 biologic repeats) (H), MMP9 mRNA levels (n=3 biologic repeats) (I), and MMP-9 activity (J) were enhanced by the overexpression of STING or IRF3 or reduced by the knockdown of STING or IRF3. K, The results of a chromatin immunoprecipitation assay showing that IRF3 bound to the MMP9 promoter in unstressed macrophages. This binding was increased in cells treated with SMC DNA and was abolished by inhibiting STING expression with STING siRNA (n=4 biologic repeats). An unpaired, two-tailed _t_-test was used in (F) and (I). Two-way ANOVA with the Bonferroni post-hoc test for pairwise comparisons was used in (I). **P<0.01, ***P<0.001. Data are presented as the mean ± standard error of the mean.
Figure 7. Association of STING activation with smooth muscle cell (SMC) injury and MMP-9 production in human sporadic ascending thoracic aortic aneurysm and dissection (ATAAD) tissues.
A, Electron microscopy images of ascending thoracic aortic aneurysm (ATAA) patient tissue showing apoptotic SMC death with nuclear fragmentation, chromatin condensation, and blebbing of the cell membrane and necrotic SMC death with swollen nuclei, mitochondria, and endoplasmic reticulum. B, Western blot analysis showing increased phosphorylation of RIP3 (p-RIP3) and MLKL (p-MLKL) in the aortic wall of ATAAD patients. C, Immunostaining showing increased p-RIP3 and p-MLKL levels in SMCs (SM22-α) of ascending thoracic aortic dissection (ATAD) tissues. Insets show a higher-magnification view. D, Immunostaining showing the colocalization of STING with p-RIP3 and p-MLKL in the SMCs (SM22-α) of ATAD tissues. E, Western blot analysis showing increased MMP-9 levels in ATAAD patient tissues. F, Immunostaining showing increased MMP-9 expression in macrophages (CD68) of diseased tissues from patients with ATAD and G, the colocalization of MMP-9 with STING and IRF3 in macrophages (CD68) of diseased tissues from patients with ATAD. Insets show a higher-magnification view.
Figure 8. Attenuation of challenge-induced aortic destruction and aortic aneurysm and dissection (AAD) development in wild-type (WT) mice treated with the STING inhibitor amlexanox.
Male and female WT mice were challenged with a high-fat diet and angiotensin II (AngII; 2000 ng/min/kg) infusion and were given either amlexanox (100 mg/kg dissolved in sunflower oil) or sunflower oil (control) daily by oral gavage during the AngII infusion period. A, Excised aortas showing that amlexanox attenuated challenged-induced AAD formation. B, Aortic diameters in various segments in both male and female challenged WT mice were reduced with amlexanox treatment. Asc, ascending; Desc, descending; SR, suprarenal; IR, infrarenal. C, The overall incidence of AAD in challenged mice was reduced with amlexanox treatment. D, Kaplan-Meier survival analysis showing improved survival at 4 weeks of angiotensin II infusion in challenged mice that received amlexanox. E, The incidence of AAD in different aortic segments was significantly lower in mice treated with amlexanox. F, The overall reduction of AAD incidence was similar in male and female mice treated with amlexanox. G, Hematoxylin and eosin (H&E) staining and Verhoeff–van Gieson elastin staining showing the preservation of aortic structure and elastic lamellar architecture in mice treated with amlexanox. H, Representative immunofluorescence staining and quantification showing lower levels of phosphorylated (p)-RIP3 and p-MLKL in aortas from the challenged mice treated with amlexanox (n=4 per group) (M: media; A: adventitia). I, Representative terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining and quantification showing that SMC apoptosis was decreased with amlexanox treatment (n=4 per group) (M: media; A: adventitia). J, Representative immunofluorescence staining showing decreased MMP-9 levels in macrophages (CD68) of aortas from challenged mice treated with amlexanox (M: media; A: adventitia). K, A schematic illustration showing that STING activation promotes AAD formation. Stress in SMCs causes DNA damage and the release of DNA from the nucleus and mitochondria into the cytosol. DNA in the cytosol binds to and activates cGAS, which produces cGAMP from ATP and GTP. cGAMP binds and activates STING and subsequently TBK1, leading to necroptotic cell death. The DNA fragments from damaged SMCs are engulfed by macrophages and are converted to cGAMP. cGAMP activates STING and subsequently IRF3, which enters the nucleus, binds to the MMP9 promoter, and induces MMP-9 expression, leading to extracellular matrix destruction. An unpaired, two-tailed _t_-test was used in (B). One-way ANOVA with the Tukey post-hoc test was used for pairwise comparisons in (H) and (I). The Fisher exact test was used for (C), (E), and (F). ***P<0.001. Data are presented as the mean ± standard error of the mean.
Figure 8. Attenuation of challenge-induced aortic destruction and aortic aneurysm and dissection (AAD) development in wild-type (WT) mice treated with the STING inhibitor amlexanox.
Male and female WT mice were challenged with a high-fat diet and angiotensin II (AngII; 2000 ng/min/kg) infusion and were given either amlexanox (100 mg/kg dissolved in sunflower oil) or sunflower oil (control) daily by oral gavage during the AngII infusion period. A, Excised aortas showing that amlexanox attenuated challenged-induced AAD formation. B, Aortic diameters in various segments in both male and female challenged WT mice were reduced with amlexanox treatment. Asc, ascending; Desc, descending; SR, suprarenal; IR, infrarenal. C, The overall incidence of AAD in challenged mice was reduced with amlexanox treatment. D, Kaplan-Meier survival analysis showing improved survival at 4 weeks of angiotensin II infusion in challenged mice that received amlexanox. E, The incidence of AAD in different aortic segments was significantly lower in mice treated with amlexanox. F, The overall reduction of AAD incidence was similar in male and female mice treated with amlexanox. G, Hematoxylin and eosin (H&E) staining and Verhoeff–van Gieson elastin staining showing the preservation of aortic structure and elastic lamellar architecture in mice treated with amlexanox. H, Representative immunofluorescence staining and quantification showing lower levels of phosphorylated (p)-RIP3 and p-MLKL in aortas from the challenged mice treated with amlexanox (n=4 per group) (M: media; A: adventitia). I, Representative terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining and quantification showing that SMC apoptosis was decreased with amlexanox treatment (n=4 per group) (M: media; A: adventitia). J, Representative immunofluorescence staining showing decreased MMP-9 levels in macrophages (CD68) of aortas from challenged mice treated with amlexanox (M: media; A: adventitia). K, A schematic illustration showing that STING activation promotes AAD formation. Stress in SMCs causes DNA damage and the release of DNA from the nucleus and mitochondria into the cytosol. DNA in the cytosol binds to and activates cGAS, which produces cGAMP from ATP and GTP. cGAMP binds and activates STING and subsequently TBK1, leading to necroptotic cell death. The DNA fragments from damaged SMCs are engulfed by macrophages and are converted to cGAMP. cGAMP activates STING and subsequently IRF3, which enters the nucleus, binds to the MMP9 promoter, and induces MMP-9 expression, leading to extracellular matrix destruction. An unpaired, two-tailed _t_-test was used in (B). One-way ANOVA with the Tukey post-hoc test was used for pairwise comparisons in (H) and (I). The Fisher exact test was used for (C), (E), and (F). ***P<0.001. Data are presented as the mean ± standard error of the mean.
Figure 8. Attenuation of challenge-induced aortic destruction and aortic aneurysm and dissection (AAD) development in wild-type (WT) mice treated with the STING inhibitor amlexanox.
Male and female WT mice were challenged with a high-fat diet and angiotensin II (AngII; 2000 ng/min/kg) infusion and were given either amlexanox (100 mg/kg dissolved in sunflower oil) or sunflower oil (control) daily by oral gavage during the AngII infusion period. A, Excised aortas showing that amlexanox attenuated challenged-induced AAD formation. B, Aortic diameters in various segments in both male and female challenged WT mice were reduced with amlexanox treatment. Asc, ascending; Desc, descending; SR, suprarenal; IR, infrarenal. C, The overall incidence of AAD in challenged mice was reduced with amlexanox treatment. D, Kaplan-Meier survival analysis showing improved survival at 4 weeks of angiotensin II infusion in challenged mice that received amlexanox. E, The incidence of AAD in different aortic segments was significantly lower in mice treated with amlexanox. F, The overall reduction of AAD incidence was similar in male and female mice treated with amlexanox. G, Hematoxylin and eosin (H&E) staining and Verhoeff–van Gieson elastin staining showing the preservation of aortic structure and elastic lamellar architecture in mice treated with amlexanox. H, Representative immunofluorescence staining and quantification showing lower levels of phosphorylated (p)-RIP3 and p-MLKL in aortas from the challenged mice treated with amlexanox (n=4 per group) (M: media; A: adventitia). I, Representative terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining and quantification showing that SMC apoptosis was decreased with amlexanox treatment (n=4 per group) (M: media; A: adventitia). J, Representative immunofluorescence staining showing decreased MMP-9 levels in macrophages (CD68) of aortas from challenged mice treated with amlexanox (M: media; A: adventitia). K, A schematic illustration showing that STING activation promotes AAD formation. Stress in SMCs causes DNA damage and the release of DNA from the nucleus and mitochondria into the cytosol. DNA in the cytosol binds to and activates cGAS, which produces cGAMP from ATP and GTP. cGAMP binds and activates STING and subsequently TBK1, leading to necroptotic cell death. The DNA fragments from damaged SMCs are engulfed by macrophages and are converted to cGAMP. cGAMP activates STING and subsequently IRF3, which enters the nucleus, binds to the MMP9 promoter, and induces MMP-9 expression, leading to extracellular matrix destruction. An unpaired, two-tailed _t_-test was used in (B). One-way ANOVA with the Tukey post-hoc test was used for pairwise comparisons in (H) and (I). The Fisher exact test was used for (C), (E), and (F). ***P<0.001. Data are presented as the mean ± standard error of the mean.
Similar articles
- Ncf1 knockout in smooth muscle cells exacerbates angiotensin II-induced aortic aneurysm and dissection by activating the STING pathway.
Liu H, Yang P, Chen S, Wang S, Jiang L, Xiao X, Le S, Chen S, Chen X, Ye P, Xia J. Liu H, et al. Cardiovasc Res. 2024 Jul 31;120(9):1081-1096. doi: 10.1093/cvr/cvae081. Cardiovasc Res. 2024. PMID: 38639325 Free PMC article. - Epigenetic Induction of Smooth Muscle Cell Phenotypic Alterations in Aortic Aneurysms and Dissections.
Chakraborty A, Li Y, Zhang C, Li Y, Rebello KR, Li S, Xu S, Vasquez HG, Zhang L, Luo W, Wang G, Chen K, Coselli JS, LeMaire SA, Shen YH. Chakraborty A, et al. Circulation. 2023 Sep 19;148(12):959-977. doi: 10.1161/CIRCULATIONAHA.123.063332. Epub 2023 Aug 9. Circulation. 2023. PMID: 37555319 Free PMC article. - Effect of Ciprofloxacin on Susceptibility to Aortic Dissection and Rupture in Mice.
LeMaire SA, Zhang L, Luo W, Ren P, Azares AR, Wang Y, Zhang C, Coselli JS, Shen YH. LeMaire SA, et al. JAMA Surg. 2018 Sep 1;153(9):e181804. doi: 10.1001/jamasurg.2018.1804. Epub 2018 Sep 19. JAMA Surg. 2018. PMID: 30046809 Free PMC article. - Programmed cell death in aortic aneurysm and dissection: A potential therapeutic target.
Chakraborty A, Li Y, Zhang C, Li Y, LeMaire SA, Shen YH. Chakraborty A, et al. J Mol Cell Cardiol. 2022 Feb;163:67-80. doi: 10.1016/j.yjmcc.2021.09.010. Epub 2021 Sep 28. J Mol Cell Cardiol. 2022. PMID: 34597613 Free PMC article. Review. - Molecular mechanisms of thoracic aortic dissection.
Wu D, Shen YH, Russell L, Coselli JS, LeMaire SA. Wu D, et al. J Surg Res. 2013 Oct;184(2):907-24. doi: 10.1016/j.jss.2013.06.007. Epub 2013 Jun 29. J Surg Res. 2013. PMID: 23856125 Free PMC article. Review.
Cited by
- Extracellular Tuning of Mitochondrial Respiration Leads to Aortic Aneurysm.
Oller J, Gabandé-Rodríguez E, Ruiz-Rodríguez MJ, Desdín-Micó G, Aranda JF, Rodrigues-Diez R, Ballesteros-Martínez C, Blanco EM, Roldan-Montero R, Acuña P, Forteza Gil A, Martín-López CE, Nistal JF, Lino Cardenas CL, Lindsay ME, Martín-Ventura JL, Briones AM, Redondo JM, Mittelbrunn M. Oller J, et al. Circulation. 2021 May 25;143(21):2091-2109. doi: 10.1161/CIRCULATIONAHA.120.051171. Epub 2021 Mar 12. Circulation. 2021. PMID: 33709773 Free PMC article. - Role of macrophages in aortic dissection pathogenesis: insights from preclinical studies to translational prospective.
Li S, Fu W, Wang L. Li S, et al. Sci China Life Sci. 2024 Nov;67(11):2354-2367. doi: 10.1007/s11427-024-2693-5. Epub 2024 Sep 30. Sci China Life Sci. 2024. PMID: 39358669 Review. - Comparative Analysis of Multiple Neurodegenerative Diseases Based on Advanced Epigenetic Aging Brain.
Shi F, He Y, Chen Y, Yin X, Sha X, Wang Y. Shi F, et al. Front Genet. 2021 May 20;12:657636. doi: 10.3389/fgene.2021.657636. eCollection 2021. Front Genet. 2021. PMID: 34093653 Free PMC article. - Cyclic GMP-AMP synthase recognizes the physical features of DNA.
Dong L, Hou YR, Xu N, Gao XQ, Sun Z, Yang QK, Wang LN. Dong L, et al. Acta Pharmacol Sin. 2024 Aug 7. doi: 10.1038/s41401-024-01369-7. Online ahead of print. Acta Pharmacol Sin. 2024. PMID: 39112770 Review. - MCM8-mediated mitophagy protects vascular health in response to nitric oxide signaling in a mouse model of Kawasaki disease.
Lin M, Xian H, Chen Z, Wang S, Liu M, Liang W, Tang Q, Liu Y, Huang W, Che D, Guo C, Idiiatullina E, Fang R, Al-Azab M, Chang J, Wang R, Li X, Zuo X, Zhang Y, Zhao J, Tang Y, Jin S, He Z, Feng D, Lu L, Zhang K, Wu Y, Bai F, Lew AM, Cui J, Wu Y, Gu X, Zhang Y. Lin M, et al. Nat Cardiovasc Res. 2023 Aug;2(8):778-792. doi: 10.1038/s44161-023-00314-x. Epub 2023 Aug 11. Nat Cardiovasc Res. 2023. PMID: 39195969
References
- Minino AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1–126. - PubMed
- Guo DC, Papke CL, He R, Milewicz DM. Pathogenesis of thoracic and abdominal aortic aneurysms. Ann N Y Acad Sci. 2006;1085:339–352. - PubMed
- Holm CK, Jensen SB, Jakobsen MR, Cheshenko N, Horan KA, Moeller HB, Gonzalez-Dosal R, Rasmussen SB, Christensen MH, Yarovinsky TO, Rixon FJ, Herold BC, Fitzgerald KA, Paludan SR. Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. Nat Immunol. 2012;13:737–743. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- S10 OD018033/OD/NIH HHS/United States
- R01 HL143359/HL/NHLBI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- S10 OD025240/OD/NIH HHS/United States
- R01 HL130193/HL/NHLBI NIH HHS/United States
- R01 HL123346/HL/NHLBI NIH HHS/United States
- R01 HL127111/HL/NHLBI NIH HHS/United States
- S10 OD023469/OD/NIH HHS/United States
- R01 HL131980/HL/NHLBI NIH HHS/United States
- R01 HL118462/HL/NHLBI NIH HHS/United States
- P30 ES030285/ES/NIEHS NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous