Liquid-like droplet formation by tumor suppressor p53 induced by multivalent electrostatic interactions between two disordered domains - PubMed (original) (raw)
Liquid-like droplet formation by tumor suppressor p53 induced by multivalent electrostatic interactions between two disordered domains
Kiyoto Kamagata et al. Sci Rep. 2020.
Abstract
Early in vivo studies demonstrated the involvement of a tumor-suppressing transcription factor, p53, into cellular droplets such as Cajal and promyelocytic leukemia protein bodies, suggesting that the liquid-liquid phase separation (LLPS) might be involved in the cellular functions of p53. To examine this possibility, we conducted extensive investigations on the droplet formation of p53 in vitro. First, p53 itself was found to form liquid-like droplets at neutral and slightly acidic pH and at low salt concentrations. Truncated p53 mutants modulated droplet formation, suggesting the importance of multivalent electrostatic interactions among the N-terminal and C-terminal domains. Second, FRET efficiency measurements for the dimer mutants of p53 revealed that distances between the core domains and between the C-terminal domains were modulated in an opposite manner within the droplets. Third, the molecular crowding agents were found to promote droplet formation, whereas ssDNA, dsDNA, and ATP, to suppress it. Finally, the p53 mutant mimicking posttranslational phosphorylation did not form the droplets. We conclude that p53 itself has a potential to form droplets that can be controlled by cellular molecules and by posttranslational modifications, suggesting that LLPS might be involved in p53 function.
Conflict of interest statement
The authors declare no competing interests.
Figures
Figure 1
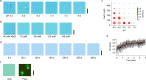
FL-p53 droplet formation is sensitive to pH and salt concentration. (a) DIC images of the FL-p53 solution in the presence of 45 mM NaCl at various pHs. (b) DIC images of the FL-p53 solution at pH 7.0 in the presence of various concentrations of NaCl. (c) 2D plot showing the pH and salt concentration dependences of FL-p53 droplet formation. The size of the circles is proportional to the OD350. The color of the circles represents the average circularity of the droplets. (d) Time course of a typical fusion event of three FL-p53 droplets into a single droplet observed at pH 7.0 in the presence of 45 mM NaCl and 150 mg/mL dextran. FL-p53 concentration was 12 μM in the experimental results shown in panels (a–d). (e) Time course of the average circularity of the fusion events. The bars denote the standard deviation of 6 fusion events. The red curve is the best-fitted curve by a single exponential. (f) DIC and fluorescence images of the p53 droplets formed by 0.12 μM Alexa488-labeled p53 and 12 μM non-labeled FL-p53 at pH 7.0 in the presence of 45 mM NaCl and 150 mg/mL dextran. Scale bars in panels (a,b,d,f) represent 10 μm.
Figure 2
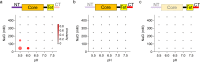
Deletion of the disordered domains hindered p53 droplet formation. The 2D plots representing droplet formation of the NTCoreTet (a), CoreTetCT (b), and TetCT mutants (c) at different pHs and salt concentrations. The size and color of the circles represent OD350 and the average circularity of the droplets, respectively. The concentration of each mutant was 12 μM. In the primary structure of mutants presented at the top of each plot, rectangles and lines denote folded and disordered domains, respectively. The shaded regions in the primary structures are the deleted domains.
Figure 3
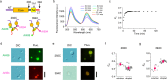
Structural characterization of the labeled dimer p53 mutants in solution and in FL-p53 droplets using FRET. (a) Schematic diagram of the primary and tertiary structures of the dimer mutant L344A. The cysteines were introduced either at position 292 or at position 394 for labeling with Alexa488 and Alexa594. (b) Fluorescence spectral changes observed after mixing the Alexa488-Alexa488 dimer and the Alexa594-Alexa594 dimer, both labeled at 292 C at pH 7.9 in the presence of 50 mM KCl. The different times describe the periods after mixing. (c) Time course of the spectrum-based FRET efficiency, _E_S, after mixing the Alexa488-Alexa488 dimer and the Alexa594-Alexa594 dimer labeled at 292 C. The data shown in panel (b) were used for _E_S calculation. (d) DIC and fluorescence microscopic images of the samples containing 0.12 μM of the Alexa488-Alexa488 dimer labeled at 292 C and 12 μM of FL-p53 (upper panels) at pH 7.0 in the presence of 45 mM NaCl and 150 mg/mL dextran. The corresponding DIC and fluorescence images prepared by adding the Alexa594-Alexa594 dimer labeled at 292 C in the same condition are shown in the lower panels. The fluorescence images were obtained through excitation at 470–490 nm for Alexa488 and at 520–550 nm for Alexa594. (e) DIC and fluorescence microscopic images of the sample after adding the Alexa488-Alexa594 dimer labeled at 292 C to the preformed FL-p53 droplets (upper panels) at pH 7.0 in the presence of 45 mM NaCl and 150 mg/mL dextran. The corresponding DIC and fluorescence images prepared by adding the Alexa488-Alexa594 dimer labeled at 394 C in the same conditions are shown in the lower panels. The fluorescence image was obtained by excitation at 470–490 nm. (f) Comparison between the apparent FRET efficiencies based on the microscopic data, _E_M, of the Alexa488-Alexa594 dimer labeled at 292 C in solution (left) and in the droplets (right). (g) Comparison between the _E_M for the Alexa488-Alexa594 dimer labeled at 394 C in solution (left) and in the droplets (right). Error bars are the standard deviation. Scale bars in panels (d,e) represent 10 μm.
Figure 4
Target binding of FL-p53 dissolved from the droplets. (a) DIC images of 12 μM FL-p53 in the presence of 45 mM NaCl and 2 mM MgCl2 at pH 5.5, 7.0, and 7.9. A scale bar represents 10 μm. (b) Fluorescence anisotropy changes for the association of FL-p53 to the specific double-stranded DNA sequence conjugated with 6-FAM. The titration of FL-p53 was conducted in the presence of 90 mM NaCl and 2 mM MgCl2 at pH 7.9 after 5-fold dilution of the FL-p53 solutions incubated in the three pH conditions shown in panel (a). Solid curves represent the best-fitted curves based on the equation assuming the one-to-one binding. (c) _K_d values of p53 for the specific double-stranded DNA sequence. The results obtained for p53 incubated at pH 5.5, 7.0 and 7.9 were compared. (d) Scattering intensity at 350 nm of the p53 solutions in the presence of 90 mM NaCl and 2 mM MgCl2 at pH 7.9 after 5-fold dilution of the FL-p53 solutions incubated in the different pHs shown in panel (a). Error bars in panels (c,d) are the fitting errors and the standard errors of three independent measurements, respectively. p53 concentrations denoted in panels (b,c) were per tetramer.
Figure 5
Molecular crowding promoted, while nucleic acids and a posttranslational modification suppressed p53 droplet formation. (a) Ficoll- and (b) Dextran-concentration dependence of p53 droplet formation detected as the scattering intensity at 350 nm in a solution containing 145 mM NaCl at pH 7.0. (c–g) DNA- and ATP-concentration dependence of p53 droplet formation detected as the scattering intensity at 350 nm in a solution containing 45 mM NaCl at pH 7.0. Panels (c–g) represent data from the addition of the specific double-stranded DNA sequence, of the non-specific double-stranded DNA, of λDNA, of single-stranded DNA, and of ATP, respectively. (h) Comparison of the effects of different DNAs in p53 droplet formation detected at pH 7.0 in the presence of 145 mM NaCl and 150 mg/mL dextran. The DNA concentration was 100 μM. Control denotes the result obtained in the absence of DNA. (i) Effect of the S392E mutation (mimicking posttranslational phosphorylation) on p53 droplet formation at pH 7.0 and in the presence of 45 mM NaCl. DIC images of FL-WT and S392E are displayed. Scale bar represents 10 μm. In panels (a–h), the error bars represent SEM of at least three measurements.
Figure 6
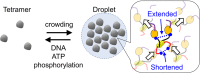
Schematic diagram of the phase separation phenomenon for p53. The LLPS of p53 is enhanced by the crowding effect. In contrast, DNA, ATP, and phosphorylation dissolve p53 droplets. p53 is composed of the NT (purple), Core (orange), Tet (yellow) and CT (red) domains. In the droplets, the NT and CT domains interact electrostatically. Arrows in the inset denote the structural changes on the different domains of p53 that are induced by the intermolecular interactions within the droplet. The dimer structure is displayed for clarity.
Similar articles
- Crystal structure of the mouse p53 core DNA-binding domain at 2.7 A resolution.
Zhao K, Chai X, Johnston K, Clements A, Marmorstein R. Zhao K, et al. J Biol Chem. 2001 Apr 13;276(15):12120-7. doi: 10.1074/jbc.M011644200. Epub 2001 Jan 4. J Biol Chem. 2001. PMID: 11152481 - Sliding of p53 along DNA can be modulated by its oligomeric state and by cross-talks between its constituent domains.
Khazanov N, Levy Y. Khazanov N, et al. J Mol Biol. 2011 Apr 29;408(2):335-55. doi: 10.1016/j.jmb.2011.01.059. Epub 2011 Feb 19. J Mol Biol. 2011. PMID: 21338609 - The Oligomerization Domains of the APC Protein Mediate Liquid-Liquid Phase Separation That Is Phosphorylation Controlled.
Bressler SG, Mitrany A, Wenger A, Näthke I, Friedler A. Bressler SG, et al. Int J Mol Sci. 2023 Mar 30;24(7):6478. doi: 10.3390/ijms24076478. Int J Mol Sci. 2023. PMID: 37047451 Free PMC article. - Signaling to p53: breaking the posttranslational modification code.
Appella E, Anderson CW. Appella E, et al. Pathol Biol (Paris). 2000 Apr;48(3):227-45. Pathol Biol (Paris). 2000. PMID: 10858956 Review. - The Tail That Wags the Dog: How the Disordered C-Terminal Domain Controls the Transcriptional Activities of the p53 Tumor-Suppressor Protein.
Laptenko O, Tong DR, Manfredi J, Prives C. Laptenko O, et al. Trends Biochem Sci. 2016 Dec;41(12):1022-1034. doi: 10.1016/j.tibs.2016.08.011. Epub 2016 Sep 23. Trends Biochem Sci. 2016. PMID: 27669647 Free PMC article. Review.
Cited by
- Profilin choreographs actin and microtubules in cells and cancer.
Pimm ML, Hotaling J, Henty-Ridilla JL. Pimm ML, et al. Int Rev Cell Mol Biol. 2020;355:155-204. doi: 10.1016/bs.ircmb.2020.05.005. Epub 2020 Jul 16. Int Rev Cell Mol Biol. 2020. PMID: 32859370 Free PMC article. - Biomolecular Condensation: A New Phase in Cancer Research.
Chakravarty AK, McGrail DJ, Lozanoski TM, Dunn BS, Shih DJH, Cirillo KM, Cetinkaya SH, Zheng WJ, Mills GB, Yi SS, Jarosz DF, Sahni N. Chakravarty AK, et al. Cancer Discov. 2022 Sep 2;12(9):2031-2043. doi: 10.1158/2159-8290.CD-21-1605. Cancer Discov. 2022. PMID: 35852417 Free PMC article. Review. - Protein of a thousand faces: The tumor-suppressive and oncogenic responses of p53.
Marques MA, de Andrade GC, Silva JL, de Oliveira GAP. Marques MA, et al. Front Mol Biosci. 2022 Aug 25;9:944955. doi: 10.3389/fmolb.2022.944955. eCollection 2022. Front Mol Biosci. 2022. PMID: 36090037 Free PMC article. Review. - Phase separation in Cancer: From the Impacts and Mechanisms to Treatment potentials.
Peng Q, Tan S, Xia L, Wu N, Oyang L, Tang Y, Su M, Luo X, Wang Y, Sheng X, Zhou Y, Liao Q. Peng Q, et al. Int J Biol Sci. 2022 Aug 1;18(13):5103-5122. doi: 10.7150/ijbs.75410. eCollection 2022. Int J Biol Sci. 2022. PMID: 35982902 Free PMC article. Review. - Single-Molecule Microscopy Meets Molecular Dynamics Simulations for Characterizing the Molecular Action of Proteins on DNA and in Liquid Condensates.
Kamagata K. Kamagata K. Front Mol Biosci. 2021 Nov 19;8:795367. doi: 10.3389/fmolb.2021.795367. eCollection 2021. Front Mol Biosci. 2021. PMID: 34869607 Free PMC article. Review.
References
- Kamagata K, Murata A, Itoh Y, Takahashi S. Characterization of facilitated diffusion of tumor suppressor p53 along DNA using single-molecule fluorescence imaging. J. Photochem. Photobiol. C Photochem. Reviews. 2017;30:36–50. doi: 10.1016/j.jphotochemrev.2017.01.004. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous