Frontline Science: Superior mouse eosinophil depletion in vivo targeting transgenic Siglec-8 instead of endogenous Siglec-F: Mechanisms and pitfalls - PubMed (original) (raw)
Frontline Science: Superior mouse eosinophil depletion in vivo targeting transgenic Siglec-8 instead of endogenous Siglec-F: Mechanisms and pitfalls
Eva Knuplez et al. J Leukoc Biol. 2020 Jul.
Abstract
Eosinophils are important multifunctional granulocytes. When studying eosinophil function and its contribution to diseases, mouse models are often used. Mouse eosinophils selectively express sialic acid-binding immunoglobulin-like lectin (Siglec)-F. Its closest functional paralog on human eosinophils is Siglec-8. These Siglecs are being used to target eosinophils when exploring their mechanistic roles in disease and for potential therapeutic benefit. In order to facilitate preclinical studies of human Siglec-8, we developed transgenic mouse strains expressing human Siglec-8 only on the surface of eosinophils with or without endogenous Siglec-F and have begun characterizing various cellular functions in vitro and in vivo. Eosinophils from Siglec-8+ mice, with or without Siglec-F, responded to Siglec-8 antibody engagement in vitro by up-regulating surface CD11b, whereas Siglec-F antibody had no such effect. Engagement of Siglec-F or Siglec-8 with respective antibodies in vitro resulted in only modest increases in cell death. Administration of rat Siglec-F antibodies to mice led to a significant decrease in Siglec-F surface expression on eosinophils due to internalization, and thus appeared to decrease eosinophil numbers based on Siglec-F+ cells, but with proper gaiting strategies did not in fact result in significant eosinophil depletion. In marked contrast, administration of mouse Siglec-8 antibodies rapidly and effectively depleted eosinophils from blood and spleens of mice, but an F(ab')2 version did not, indicating an Fc-mediated mechanism for eosinophil depletion in vivo. Siglec-8 expressing mice with or without endogenous Siglec-F will be useful to study Siglec-8-based therapeutics, and may be a preferred approach when acute or chronic eosinophil depletion is needed.
Keywords: Siglec-8; Siglec-F; antibody-dependent cellular cytotoxicity; depletion; eosinophils.
©2020 Society for Leukocyte Biology.
Conflict of interest statement
Disclosure
B.S.B. receives remuneration for serving on the scientific advisory board of Allakos, Inc. and owns stock in Allakos. He receives publication-related royalty payments from Elsevier and UpToDate®. He is a co-inventor on existing Siglec-8–related patents and thus may be entitled to a share of royalties received by Johns Hopkins University during development and potential sales of such products. Dr. Bochner is also a co-founder of Allakos, which makes him subject to certain restrictions under University policy. The terms of this arrangement are being managed by Johns Hopkins University and Northwestern University in accordance with their conflict of interest policies. The other authors have no competing financial interests.
Figures
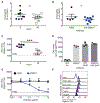
Figure 1.
Phenotypic characterization of bone marrow derived eosinophils. Eosinophils from mice of different genetic backgrounds were differentiated from bone marrow hematopoietic stem cells according to protocol presented in (A). Surface expression of Siglec-8, Siglec-F and CCR3 was followed over time by flow cytometry in differentiating cells from WT (B), Siglec8+F+ (C), Siglec8+F− (D), SiglecF−/− (E) mice. Data from 4-5 mice per genetic model are shown. (F) Mouse Siglec8+F− eosinophils stained with Diff-Qwik on day 14 of differentiation. Siglec-F (G-H) and Siglec-8 (I-J) expression on mouse eosinophils was confirmed and quantified in peripheral blood and spleen of animals. Data from 2- 6 mice per genetic model are shown.
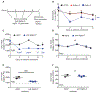
Figure 2.
CD11b expression on bone marrow-derived eosinophils following Siglec antibody engagement. Mature bone marrow derived eosinophils were primed with 30 ng/mL of recombinant mouse IL-5 for 4 h and afterwards incubated with vehicle or 2.5 μg/mL of isotype control (mIgG1), anti Siglec-8 (2C4 or 2E2) or anti Siglec-F (E50-2440) for 2 h. CD11b expression was evaluated by flow cytometry and normalized to vehicle control. (A) WT eosinophils, (B) SiglecF−/− eosinophils, (C) Siglec8+F+ eosinophils and (D) Siglec8+F− eosinophils. Data from 4-5 biological replicates per genetic model are presented and analyzed with one-way ANOVA followed by Dunnett’s posttest (antibody treated versus isotype control).
Figure 3.
Effect of Siglec engagement in vitro on eosinophil viability. Cell viability of mature bone marrow eosinophils was assessed with Annexin V / DAPI staining. (A) WT eosinophils were pretreated with isotype control or anti Siglec-F (E50–2440, 1.25 – 10 μg/mL) for 48 h. (B) Representative histograms of Annexin V / DAPI staining of eosinophils in (A) treated with vehicle or isotype control and anti Siglec-F at 10 μg/mL. (C) Siglec8+F+ eosinophils were pretreated with 1.25 – 10 μg/mL isotype control, anti Siglec-F (E50-2440) or anti Siglec-8 (2C4) for 48 h. Data from 4 independent experiments performed in duplicates are shown and analyzed with two-way ANOVA followed by Sidak’s posttest. ** p<0.01 anti Siglec-F versus isotype control; * p<0.05 anti Siglec-F versus isotype control, # p<0.05 anti Siglec-8 versus isotype control. (D) Siglec8+F− mouse eosinophils were pretreated with 10 μg/mL isotype controls (mouse IgG1 or rat IgG2), anti Siglec-8 (2C4) or anti Siglec-F (E50-2440 or 238047) for 24 h. (E) Siglec8+F− mouse eosinophils were pretreated with 10 μg/mL saporin-conjugated isotype controls (mouse IgG1 or rat IgG2), anti Siglec-8 (2C4) or anti Siglec-F (9C7) antibodies for 24 h
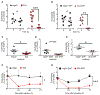
Figure 4.
Single administration of Siglec-F mAb in vivo decreases the surface expression of Siglec-F on eosinophils, but does not effectively deplete eosinophils. Siglec8+F+ mice received a single i.p. injection of 15 μg of isotype control or anti-Siglec F (238047). (A) Percentage of eosinophils (analyzed as % of CD45+ cells, see representative gating strategy in Supplementary Figure 1) at baseline and 48 h after the administration of anti Siglec-F. (B) Eosinophil percentage in blood of isotype treated compared to anti Siglec-F treated animals. Data from 2 independent experiments are shown. n=5-9, ns (non-significant), unpaired Student’s t-test. (C) Geometric mean of Siglec-F PE (clone E50-2440 used for detection) on blood eosinophils before and 48 h after anti Siglec-F (clone 238047) administration. (D) Additivity trial of two Siglec-F mAb clones. Following red blood cell lysis, mouse blood leukocytes were incubated with vehicle or anti Siglec-F (clone 238047 or clone E50-2440, 5 μg/mL) or both (5 μg/mL each). A secondary FITC-labelled polyclonal anti-rat IgG was then added to detect bound primary antibodies. 2nd Ab=vehicle treated cells incubated only with secondary antibody. (E-F) Following red blood cell lysis, mouse blood leukocytes were incubated with isotype control or anti Siglec-F (clone 238047) at the indicated concentrations for 20 min, washed, and then incubated with a different anti Siglec-F-PE clone. (E) Geometric mean of Siglec-F PE (staining clone E50-2440) is quantified with representative histograms of anti Siglec-F pretreated cells shown in (F). Mean ± SEM from 2-3 independent experiments is shown. Data were analyzed with two-way ANOVA followed by Sidak’s posttest.*** p<0.001 anti Siglec-F (238047) versus isotype treated cells.
Figure 5.
Siglec-8 is a more reliable marker for detecting eosinophils than Siglec-F following systemic administration of Siglec-F antibody. (A) protocol used for the prolonged model of anti Siglec-F treatment. (B) Eosinophil percentage in blood samples as determined by Siglec-F, Siglec-8 or CCR3 positivity over time. Mean ± SEM of 3 biologic replicates from 1-2 independent experiments are shown. **p<0.01 Siglec-F versus CCR3 as a marker, two-way ANOVA with Dunnett’s posttest. (C-D) Geometric mean of Siglec-F-PE or Siglec-8-AF647 labeling of eosinophils in isotype versus anti Siglec-F treated animals over time. ns=non-significant, *p<0.05, **p<0.01, ***p<0.001 analyzed with two-way ANOVA followed with Sidak’s posttest. (E-F) Geometric mean of Siglec-F-PE or Siglec-8-AF647 labeling of eosinophils from spleens of isotype and anti Siglec-F treated animals. Data from 1-2 independent experiments are shown and analyzed with unpaired Student’s t-test. **p<0.01, ns (non-significant, p>0.05).
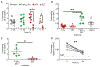
Figure 6.
A single dose of 2C4 or 2C4-saporin effectively depletes eosinophils in vivo. A single application of anti Siglec-8 (with or without saporin) effectively depletes eosinophils in vivo. (A-B) Eosinophil percentages at baseline and 24 h following a single i.p. injection of 10 μg anti Siglec-8 (2C4 or 2C4-saporin) or isotype control (mIgG1 or mIgG1-saporin). Data from 5-7 mice per treatment are shown and analyzed with two-way ANOVA followed by Sidak’s posttest (0 h versus 24 h). Eosinophil percentages in spleen (C-D) and bone marrow (E) 24 h after treatment are shown and compared to isotype control with unpaired Student’s t-test. (F-G) Duration of anti Siglec-8-mediated eosinophil depletion was followed with repeated blood sampling and is represented as eosinophil percentages in blood over time. Means ± SEM of 3 mice per treatment group are shown and analyzed with two-way ANOVA followed by Dunnett’s posttest (anti Siglec-8 versus isotype control treated mice).
Figure 7.
Repeated dosing of 2C4 mAb effectively depletes eosinophils in vivo from multiple body compartments. (A) protocol used for anti Siglec-8 (2C4) treatment. (B) Eosinophil percentage in blood samples as determined by Siglec-F, Siglec-8 or CCR3 positivity over time. Mean ± SEM of 3 biologic replicates. Two-way ANOVA with Sidak’s posttest. ***p<0.001 CCR3+ population versus time zero baseline, §§§ p<0.001 Siglec-F+ population versus time zero baseline, ### p<0.001 Siglec-8+ population versus time zero baseline. (C) Percentage of eosinophils in bone marrow on day 6 of the protocol. (D) Percentage of eosinophils in spleen on day 6 of the protocol. Differences between isotype treated animals (n=2) and 2C4 treated animals (n=3) were evaluated with unpaired Student’s t-test.
Figure 8.
The effect of 2C4-mediated eosinophil depletion in vivo is dependent on the Fc region of the mIgG1. Siglec8+F+ mice were given a single i.p. injection of 10 μg isotype control, anti Siglec-8 (2C4 or 2E2) or the F(ab’)2 fragment of 2E2. (A) Eosinophil percentages (CCR3+Siglec-F+) were determined from blood samples at baseline and 24 h after treatment. Data from 5-7 biological replicates per treatment are shown and analyzed with two-way ANOVA followed with Sidak’s posttest (baseline vs 24 h). (B) Eosinophil percentages in spleen 24 h after treatment are presented. Data are analyzed by one-way ANOVA followed by Tukey’s posttest. (C) Eosinophil percentages in blood of 2E2 and F(ab’)2 2E2 treated animals are shown and compared with unpaired Student’s t-test. (D) Geometric mean of Siglec-8-AF647 (1H10 staining clone) labeling of eosinophils at baseline and 24 h following treatment with non-cross-reactive 2E2 F(ab’)2. Data from 5 biologic replicates are shown and analyzed with paired Student’s t-test.
Comment in
- Divergent Siglec-F(eights) of mouse and human eosinophil death.
Jacobsen EA. Jacobsen EA. J Leukoc Biol. 2020 Jul;108(1):9-11. doi: 10.1002/JLB.5CE0520-108R. Epub 2020 Jun 18. J Leukoc Biol. 2020. PMID: 32557797 Free PMC article.
Similar articles
- Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors.
Bochner BS. Bochner BS. Clin Exp Allergy. 2009 Mar;39(3):317-24. doi: 10.1111/j.1365-2222.2008.03173.x. Clin Exp Allergy. 2009. PMID: 19178537 Free PMC article. Review. - Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils.
Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Zhang M, et al. Blood. 2007 May 15;109(10):4280-7. doi: 10.1182/blood-2006-08-039255. Epub 2007 Feb 1. Blood. 2007. PMID: 17272508 Free PMC article. - Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils.
Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, Crocker PR, Rothenberg ME, Bochner BS. Zimmermann N, et al. Allergy. 2008 Sep;63(9):1156-63. doi: 10.1111/j.1398-9995.2008.01709.x. Allergy. 2008. PMID: 18699932 Free PMC article. - Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions.
Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Kiwamoto T, et al. Pharmacol Ther. 2012 Sep;135(3):327-36. doi: 10.1016/j.pharmthera.2012.06.005. Epub 2012 Jun 27. Pharmacol Ther. 2012. PMID: 22749793 Free PMC article. Review.
Cited by
- In vivo edited eosinophils reconcile antigen specific Th2 response and mitigate airway allergy.
Luo X, Yang J, Zheng H, Zhang Y, Mo L, Huang Q, Wu G, Zhong J, Liu Y, Yang G, Yang P. Luo X, et al. Cell Commun Signal. 2024 Sep 30;22(1):462. doi: 10.1186/s12964-024-01824-2. Cell Commun Signal. 2024. PMID: 39350231 Free PMC article. - Stress-free single-cell transcriptomic profiling and functional genomics of murine eosinophils.
Borrelli C, Gurtner A, Arnold IC, Moor AE. Borrelli C, et al. Nat Protoc. 2024 Jun;19(6):1679-1709. doi: 10.1038/s41596-024-00967-3. Epub 2024 Mar 19. Nat Protoc. 2024. PMID: 38504138 Review. - Siglecs as potential targets of therapy in human mast cell- and/or eosinophil-associated diseases.
O'Sullivan JA, Youngblood BA, Schleimer RP, Bochner BS. O'Sullivan JA, et al. Semin Immunol. 2023 Sep;69:101799. doi: 10.1016/j.smim.2023.101799. Epub 2023 Jul 4. Semin Immunol. 2023. PMID: 37413923 Free PMC article. Review. - Siglecs in allergy and asthma.
Bochner BS, O'Sullivan JA, Chang AT, Youngblood BA. Bochner BS, et al. Mol Aspects Med. 2023 Apr;90:101104. doi: 10.1016/j.mam.2022.101104. Epub 2022 Jul 11. Mol Aspects Med. 2023. PMID: 35835621 Free PMC article. Review. - Siglec-F Promotes IL-33-Induced Cytokine Release from Bone Marrow-Derived Eosinophils Independently of the ITIM and ITIM-like Motif Phosphorylation.
Westermann S, Dietschmann A, Doehler D, Castiglione K, Bochner BS, Voehringer D, Radtke D. Westermann S, et al. J Immunol. 2022 Feb 1;208(3):732-744. doi: 10.4049/jimmunol.2100184. Epub 2022 Jan 7. J Immunol. 2022. PMID: 34996839 Free PMC article.
References
- Lee JJ, Dimina D, Macias MP, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. - PubMed
- Humbles AA, Lloyd CM, McMillan SJ, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials