Interplay Between Human Gut Bacteria Escherichia coli and Lactobacillus mucosae in the Occurrence of Neuropsychiatric Disorders in Mice - PubMed (original) (raw)
Interplay Between Human Gut Bacteria Escherichia coli and Lactobacillus mucosae in the Occurrence of Neuropsychiatric Disorders in Mice
Jeon-Kyung Kim et al. Front Immunol. 2020.
Abstract
To understand the roles of human gut bacteria in the occurrence of neuropsychiatric disorders, we isolated inflammatory Escherichia coli K1 and anti-inflammatory Lactobacillus mucosae from healthy human feces and examined their effects on the occurrence of altered microbiota, cognitive decline, and depression in mice. Oral gavage of Escherichia coli K1 caused colitis, cognitive decline, and depression in mice in the elevated plus maze, tail suspension, and forced swimming tasks. However, NK41 treatment reduced K1-induced cognitive decline and anxiety/depression. Furthermore, NK41 treatment increased K1-suppressed brain-derived neurotrophic factor (BDNF) expression and BDNF+/NeuN+ cell population and suppressed K1-induced NF-κB activation and LPS+/Iba1+ and NF-κB+/Iba1+ (microglial) cell populations in the hippocampus. NK41 treatment also suppressed K1-induced TNF-α and LPS levels in the blood and TNF-α expression, myeloperoxidase activity, NF-κB+/CD11c+ and CD11b+/CD11c+ cell populations in the colon. Furthermore, NK41 treatment decreased K1-induced colonic MUC2 expression, gut Proteobacteria population, and fecal LPS levels and modified the bacterial abundance related to polysaccharide breaking and biosynthesis. In conclusion, the overgrowth of inflammatory bacteria such as Escherichia coli in the gastrointestinal tract can cause neuropsychiatric disorders with gut microbiota alteration and the superiority of anti-inflammatory bacteria such as Lactobacillus mucosae can alleviate neuropsychiatric disorders with the attenuation of altered microbiota.
Keywords: brain; colon; gut bacteria; inflammation; neuropsychiatric disorder.
Copyright © 2020 Kim, Lee, Lee, Jang and Kim.
Figures
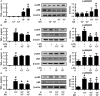
Figure 1
Effects of gut bacteria K1 and NK41 on the TNF-α expression and NF-κB activation in macrophages. (A) Effects of NK41 and K1 on TNF-α expression and NF-κB activation in macrophages. (B) Effects of NK41 on TNF-α expression and NF-κB activation in LPS-stimulated macrophages. (C) Effect of K1 on TNF-α expression and NF-κB activation in LPS-stimulated macrophages. (D) Effect of NK41 on NF-κB activation in K1-stimulated macrophages. Macrophage cells (1 × 106/mL) were incubated with K1 or NK41 (1 × 103 or 1 × 105 CFU/mL) in the absence or presence of LPS for 2 h (for NF-κB) or 20 h (for TNF-α). p-p65 and p65 (NF-κB) were measured by immunoblotting. TNF-α was measured by ELISA kit. Data values are indicated as mean ± SD (n = 4). #p < 0.05 vs. Con group treated with Vehicle alone. *p < 0.05 vs. group treated with K1 and LPS alone.
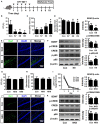
Figure 2
Effects of K1 and NK41 on the occurrence of neuropsychiatric disorders in mice. (A) Experimental protocol. (B) Effect of K1 on the time spent in open arms (OT) in EPM task. (C) Effect of K1 on the immobility in the forced swimming task. (D) Effect of K1 on memory impairment in Y-maze task. (E) Effect of K1 on the infiltration of Iba1+ cells into the hippocampus. (F) Effect of K1 on BDNF expression, CREB phosphorylation, and NF-κB activation in the hippocampus. (G) Effect of NK41 on the depression in EPM task. Effect of NK41 on the cogntive decline in the Y-maze (H) and Banes maze tasks (I). (J) Effect of NK41 on the infiltration of Iba1+ cells into the hippocampus. (K) Effect of NK41 on BDNF expression, CREB phosphorylation, and NF-κB activation in the hippocampus. Mice were exposed to K1 or NK41 (C, vehicle [1% maltose]; K7, 1 × 107 CFU/mouse/day of K1; K8, 1 × 108 CFU/mouse/day of K1; K9, 1 × 109 CFU/mouse/day of K1; NK8, 1 × 108 CFU/mouse/day of NK41; or NK9, 1 × 109 CFU/mouse/day of NK41) daily for 5 days and thereafter treated with vehicle for 5 days. Normal control group (Con), not exposed to gut bacteria, was treated with 1% maltose instead of gut bacteria. Data values were indicated as mean ± SD (n = 7). *p < 0.05 vs. Con group.
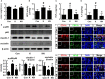
Figure 3
NK41 suppressed K1-induced altered microbiota in the feces mice. Effects on the composition of gut microbiota, analyzed by the pyrosequencing: phylum (A), principal coordinate analysis (PCoA) plot based on weighted pairwise Fast UniFrac analysis (B), and OTUs and Shannon (C). (D) Cladogram generated by LEfSE indicating significant differences in gut microbial (family) abundances among Con (blue), NK (purple), K (red), and KN (green) groups. Yellow nodes represent species with no significant difference. The threshold logarithmic score set at 2.0 in the family level and ranked. (E) Effects on fecal Escherichia coli and Lactobacillus mucosae, assessed by qPCR. (F) Effects on the fecal LPS level. LPS levels were assayed by ELISA kits. (G) The abundance of bacterial genes predicted using the method of PICRUSt. The difference was analyzed using the Kruskal-Wallis H test. (H) Effects on the MUC1 and MUC2 expression in the colon. (I) Histological examination of colons, stained with alcian blue. NK and K groups were exposed to Lactobacillus mucosae NK41 (1 × 109 CFU/mouse/day of NK41) and Escherichia coli K1 (1 × 109 CFU/mouse/day) daily for 5 days, respectively, and thereafter treated with vehicle (1% maltose) daily for 5 days. KN group was exposed to Escherichia coli K1 (1 × 109 CFU/mouse/day) daily for 5 days and thereafter treated with Lactobacillus mucosae NK41 (1 × 109 CFU/mouse/day of NK41) daily for 5 days. Con group was treated with vehicle instead of gut bacteria. Data values were indicated as mean ± SD (n = 5). #p < 0.05 vs. Con group. *p < 0.05 vs. K group.
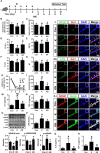
Figure 4
NK41 signiticantly suppressed K1-induced gut inflammation in mice. Effects on colon length (A), myeloperoxidase (MPO) activity (B), and TNF-α (C) and IL-6 (D) expression in the colon. (E) Effects on occludin and claudin-1 expression and NF-kB activation (E) in the colon. Effects on CD11b+/CD11c+ (F) and NF-κB+/CD11c+ cell populations (G) in the colon. NK and K groups were exposed to Lactobacillus mucosae NK41 (1 × 109 CFU/mouse/day of NK41) and Escherichia coli K1 (1 × 109 CFU/mouse/day) daily for 5 days, respectively, and thereafter treated with vehicle (1% maltose) daily for 5 days. KN group was exposed to Escherichia coli K1 (1 × 109 CFU/mouse/day) daily for 5 days and thereafter treated with Lactobacillus mucosae NK41 (1 × 109 CFU/mouse/day of NK41) daily for 5 days. Con group was treated with vehicle instead of gut bacteria. Colonic p65, p-p65, and β-actin were analyzed by immunoblotting. TNF-α and IL-6 levels were assayed by ELISA kits. NF-κB+, CD11b+, and CD11c+ cells were measured using a confocal microscope. Arrows indicate postive cells. Data values were indicated as mean ± SD (n = 7). #p < 0.05 vs. Con group. *p < 0.05 vs. K group.
Figure 5
NK41 signiticantly suppressed K1-induced neuropsychiatric disorders in mice. (A) Experimental protocol. Effect on the cognition function in Y-maze (B), NOR (C), and Banes maze (D). Effect on the depressive behaviors in the forced swimming (E), EPM (F: OT, time spent in open arms; G: OE, open arm entries), and light-dark transition tasks (H: TL, time spent in the light dark compartment; I: NT, number of transitions into the light dark compartment). Effect on the infitration of NF-κB+/Iba1+ (J), LPS+/Iba1+ (K), and BDNF+/NeuN+ cells (L) into the hippocampus. Effects on IL-6 (M), TNF-α (N), and BDNF expression, CREB phosphorylation, and NF-κB activation (O). Effects on the LPS (P), IL-6 (Q), and TNF-α levels (R) in the blood. NK and K groups were exposed to Lactobacillus mucosae NK41 (1 × 109 CFU/mouse/day of NK41) and Escherichia coli K1 (1 × 109 CFU/mouse/day) daily for 5 days, respectively, and thereafter treated with vehicle (1% maltose) daily for 5 days. KN group was exposed to Escherichia coli K1 (1 × 109 CFU/mouse/day) daily for 5 days and thereafter treated with Lactobacillus mucosae NK41 (1 × 109 CFU/mouse/day of NK41) daily for 5 days. Con group was treated with vehicle instead of gut bacteria. TNF-α, IL-6, and LPS were assayed by ELISA. p65, p-p65, CREB, p-CREB, BDNF, and β-actin were analyzed by immunoblotting. Iba1+, NF-κB+, LPS+ and NeuN+ cells were measured using a confocal microscope). #p < 0.05 vs. Con group. *p < 0.05 vs. K group.
Figure 6
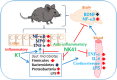
Interplay between Escherichia coli K1 and Lactobacillus mucosae NK41 on the occurrence of neuropsychotric disorders and altered microbiota.
Similar articles
- Alleviation of Cognitive Impairment-like Behaviors, Neuroinflammation, Colitis, and Gut Dysbiosis in 5xFAD Transgenic and Aged Mice by Lactobacillus mucosae and Bifidobacterium longum.
Ma X, Kim JK, Shin YJ, Son YH, Lee DY, Park HS, Kim DH. Ma X, et al. Nutrients. 2023 Jul 29;15(15):3381. doi: 10.3390/nu15153381. Nutrients. 2023. PMID: 37571319 Free PMC article. - A Probiotic Lactobacillus gasseri Alleviates _Escherichia coli_-Induced Cognitive Impairment and Depression in Mice by Regulating IL-1β Expression and Gut Microbiota.
Yun SW, Kim JK, Lee KE, Oh YJ, Choi HJ, Han MJ, Kim DH. Yun SW, et al. Nutrients. 2020 Nov 10;12(11):3441. doi: 10.3390/nu12113441. Nutrients. 2020. PMID: 33182607 Free PMC article. - Lacticaseibacillus paracasei NK112 mitigates _Escherichia coli_-induced depression and cognitive impairment in mice by regulating IL-6 expression and gut microbiota.
Yun SW, Kim JK, Han MJ, Kim DH. Yun SW, et al. Benef Microbes. 2021 Nov 16;12(6):541-551. doi: 10.3920/BM2020.0109. Epub 2021 Sep 13. Benef Microbes. 2021. PMID: 34511050 - The role of prebiotics in cognition, anxiety, and depression.
Paiva IHR, Duarte-Silva E, Peixoto CA. Paiva IHR, et al. Eur Neuropsychopharmacol. 2020 May;34:1-18. doi: 10.1016/j.euroneuro.2020.03.006. Epub 2020 Mar 30. Eur Neuropsychopharmacol. 2020. PMID: 32241688 Review. - Escherichia coli Residency in the Gut of Healthy Human Adults.
Martinson JNV, Walk ST. Martinson JNV, et al. EcoSal Plus. 2020 Sep;9(1):10.1128/ecosalplus.ESP-0003-2020. doi: 10.1128/ecosalplus.ESP-0003-2020. EcoSal Plus. 2020. PMID: 32978935 Free PMC article. Review.
Cited by
- Alleviation of Immobilization Stress or Fecal Microbiota-Induced Insomnia and Depression-like Behaviors in Mice by Lactobacillus plantarum and Its Supplement.
Lee DY, Baek JS, Shin YJ, Kim DH. Lee DY, et al. Nutrients. 2024 Oct 30;16(21):3711. doi: 10.3390/nu16213711. Nutrients. 2024. PMID: 39519543 Free PMC article. - The extracellular vesicle of depressive patient-derived Escherichia fergusonii induces vagus nerve-mediated neuroinflammation in mice.
Ma X, Park HS, Shin YJ, Kim JK, Hong JK, Han SW, Yoon IY, Kim DH. Ma X, et al. J Neuroinflammation. 2024 Sep 14;21(1):224. doi: 10.1186/s12974-024-03211-7. J Neuroinflammation. 2024. PMID: 39277768 Free PMC article. - Functional Effects of Probiotic Lactiplantibacillus plantarum in Alleviation Multidrug-Resistant Escherichia coli-Associated Colitis in BALB/c Mice Model.
Ismael M, Qayyum N, Gu Y, Na L, Haoyue H, Farooq M, Wang P, Zhong Q, Lü X. Ismael M, et al. Probiotics Antimicrob Proteins. 2024 Sep 13. doi: 10.1007/s12602-024-10356-7. Online ahead of print. Probiotics Antimicrob Proteins. 2024. PMID: 39271561 - Heat-Processed Soybean Germ Extract and Lactobacillus gasseri NK109 Supplementation Reduce LPS-Induced Cognitive Impairment and Colitis in Mice.
Yun SW, Lee DY, Park HS, Kim DH. Yun SW, et al. Nutrients. 2024 Aug 16;16(16):2736. doi: 10.3390/nu16162736. Nutrients. 2024. PMID: 39203872 Free PMC article. - Investigations of microbiota composition and neuroactive pathways in association with symptoms of stress and depression in a cohort of healthy women.
Bashir Z, Hugerth LW, Krog MC, Prast-Nielsen S, Edfeldt G, Boulund F, Schacht SR, Tetens I, Engstrand L, Schuppe-Koistinen I, Fransson E, Nielsen HS. Bashir Z, et al. Front Cell Infect Microbiol. 2024 Jul 2;14:1324794. doi: 10.3389/fcimb.2024.1324794. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39015337 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous