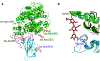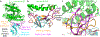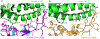Structural basis of receptor recognition by SARS-CoV-2 - PubMed (original) (raw)
Comparative Study
Structural basis of receptor recognition by SARS-CoV-2
Jian Shang et al. Nature. 2020 May.
Abstract
A novel severe acute respiratory syndrome (SARS)-like coronavirus (SARS-CoV-2) recently emerged and is rapidly spreading in humans, causing COVID-191,2. A key to tackling this pandemic is to understand the receptor recognition mechanism of the virus, which regulates its infectivity, pathogenesis and host range. SARS-CoV-2 and SARS-CoV recognize the same receptor-angiotensin-converting enzyme 2 (ACE2)-in humans3,4. Here we determined the crystal structure of the receptor-binding domain (RBD) of the spike protein of SARS-CoV-2 (engineered to facilitate crystallization) in complex with ACE2. In comparison with the SARS-CoV RBD, an ACE2-binding ridge in SARS-CoV-2 RBD has a more compact conformation; moreover, several residue changes in the SARS-CoV-2 RBD stabilize two virus-binding hotspots at the RBD-ACE2 interface. These structural features of SARS-CoV-2 RBD increase its ACE2-binding affinity. Additionally, we show that RaTG13, a bat coronavirus that is closely related to SARS-CoV-2, also uses human ACE2 as its receptor. The differences among SARS-CoV-2, SARS-CoV and RaTG13 in ACE2 recognition shed light on the potential animal-to-human transmission of SARS-CoV-2. This study provides guidance for intervention strategies that target receptor recognition by SARS-CoV-2.
Figures
Extended Data Figure 1:
Sequence alignment of the RBDs from SARS-CoV and SARS-like viruses. RBM is in purple. Previously identified critical ACE2-binding residues are in blue. The seven RBM residues that differ between SARS-CoV-2 wild-type RBD and SARS-CoV-2 chimeric RBD are shaded. A critical arginine on the side loop of SARS-CoV RBM that forms a strong salt bridge with human ACE2 is in green. Another arginine in the core structure that interacts with glycan is in cyan. The residues on the variable loop between two disulfide-bond-forming cysteines in the ACE2-binding ridge are in red. The significant motif changes in the ACE2-binding ridge are underlined. GenBank accession numbers are: QHD43416.1 for SARS-CoV-2 spike; AFR58742 for SARS-CoV spike; AY304486.1 for civet SARS-CoV spike; MG916901.1 for bat Rs3367 spike; QHR63300.2 for bat RaTG13 spike. Two coronaviruses, CoV-pangolin/GD and CoV-pangolin/GX, were isolated from pangolins at two different locations in China, Guangdong (GD) and Guangxi (GX); their RBD sequences were from reference .
Extended Data Figure 2:
Interface between SARS-CoV-2 or SARS-CoV RBM and human ACE2. (a) Interface between SARS-CoV RBD and human ACE2, showing a strong salt bridge between Arg426 on the side loop in the RBM and Glu329 from human ACE2. Core structure is in grey. RBM is in orange. (b) Interface between SARS-CoV-2 chimeric RBD and human ACE2, showing a weaker, but still energetically favorable, N-O bridge between Arg439 on the side loop in the RBM and Glu329 from human ACE2. The interaction between Arg439 on the side loop in the RBM and Glu329 from human ACE2 is non-natural in SARS-CoV-2 (i.e., resulting from the design of the SARS-CoV-based chimera).
Extended Data Figure 3:
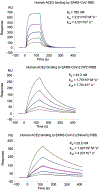
Comparison of human ACE2 binding by SARS-CoV RBD, SARS-CoV-2 wild-type RBD, and SARS-CoV-2 chimeric RBD. (a) Buried surface areas at SARS-CoV RBM/human ACE2 and SARS-CoV-2 RBM/human ACE2 interfaces. In the crystals for both SARS-CoV RBD/ACE2 complex and chimeric RBD/ACE2 complex, two copes of each complex were present in one asymmetric unit. Numbers for both copies of the complexes are shown. The interaction between Arg439 on the side loop in the RBM and Glu329 from human ACE2 was excluded in the calculation of buried surface area for SARS-CoV-2. (b) List of contact residues from RBM and ACE2 that are directly involved in RBM/ACE2 binding. The engineered Arg439 in the chimeric RBD is in orange. Contact residues from SARS-CoV RBM/ACE2 are taken from PDB 2AJF. (c) Binding affinities between the RBDs and human ACE2 as measured using surface plasmon resonance.
Extended Data Figure 4:
Composite omit map of the interface between SARS-CoV-2 RBM and human ACE2. Contour level is 1 sigma.
Extended Data Figure 5:
Glycans built into the SARS-CoV-2 chimeric RBD/hACE2 structure. (A) Distribution of glycans in the structure. Glycans are in red. The residues that the glycans attach to are in parentheses. (B) Interaction between a glycan attached the ACE2 residue 90 and Arg408 from the RBD.
Extended Data Figure 6:
Measurement of binding affinities between RBDs and human ACE2 by surface plasmon resonance assay using Biacore. Purified recombinant RBDs were covalently immobilized to the sensor chip via their amine groups, and purified recombinant hACE2 flowed by. Here hACE2 was diluted to different concentrations (from 5 to 80 nM for SARS-CoV-2 RBD and chimeric RBD, and 20-320 nM for SARS-CoV RBD) before being injected. The resulting data were fit to a 1:1 binding model. Each experiment was repeated independently twice with similar results. Each time, five different protein concentrations were used to calculate the Kd values.
Extended Data Figure 7:
Summary of human ACE2 adaptation and evolution of SARS-CoV-2.
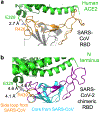
Figure 1:. Structure of SARS-CoV-2 chimeric RBD complexed with human ACE2.
(a) Crystal structure of SARS-CoV-2 chimeric RBD complexed with ACE2. ACE2 is in green. RBD core is in cyan. RBM is in magenta. A side loop in RBM is in orange. A zinc ion in ACE2 is in blue. (b) Comparison of the conformations of the ridge in SARS-CoV-2 RBM (purple) and SARS-CoV RBM (orange). (c) Comparison of the conformations of the ridge from another angle of view. In SARS-CoV RBM, a proline-proline-alanine motif is shown. In SARS-CoV-2 RBM, a newly formed hydrogen bond, Phe486, and some of the ridge’s interactions with the N-terminal helix of ACE2 are shown.
Figure 2:. Structural details at the interface between SARS-CoV-2 RBM and human ACE2.
(A) Interface between SARS-CoV-2 RBM and human ACE2. (B) Interface between SARS-CoV RBM and human ACE2.
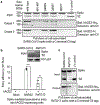
Figure 3:. Biochemical data showing the interactions between SARS-CoV-2 or bat RaTG13 spike and human ACE2.
(A) Protein pull-down assay using hACE2 as the bait and cell-associated SARS-CoV-2 spike molecules (wild type and mutants) as the targets. Top panel: cell-expressed SARS-CoV-2 spike. Middle panel: pull-down result using His6-tagged hACE2. Bottom panel: pull-down result using Fc-tagged hACE2. MERS-CoV spike was used as a negative control. (B) Entry of SARS-CoV-2 and bat RaTG13 pseudoviruses into hACE2-expressing cells. Top: packaged SARS-CoV-2 and bat RaTG13 pseudoviruses. HIV p24 was detected as an internal control. Bottom: pseudovirus entry efficiency. Mock: no pseudoviruses. Error bars indicate +1 S.D. Two-tailed t-test comparing SARS-CoV-2 (with hACE2; _n_=3 independent samples) with SARS-CoV-2 (no hACE2; _n_=4 independent samples) showed a significant difference, p<1.16×10−8. Two-tailed t-test comparing RaTG13 (with hACE2; _n_=3 independent samples) with RaTG13 (no hACE2; _n_=4 independent samples) showed a significant difference, _p_=.0097. Individual data points are in black dots. *** p<.001. ** p<.01.(C) Protein pull-down assay using hACE2 as the bait and cell-associated RaTG13 spike as the target. All experiments were repeated independently three times with similar results.
Similar articles
- Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2.
Liu Z, Xiao X, Wei X, Li J, Yang J, Tan H, Zhu J, Zhang Q, Wu J, Liu L. Liu Z, et al. J Med Virol. 2020 Jun;92(6):595-601. doi: 10.1002/jmv.25726. Epub 2020 Mar 11. J Med Virol. 2020. PMID: 32100877 Free PMC article. - Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus.
Wan Y, Shang J, Graham R, Baric RS, Li F. Wan Y, et al. J Virol. 2020 Mar 17;94(7):e00127-20. doi: 10.1128/JVI.00127-20. Print 2020 Mar 17. J Virol. 2020. PMID: 31996437 Free PMC article. - Role of the GTNGTKR motif in the N-terminal receptor-binding domain of the SARS-CoV-2 spike protein.
Behloul N, Baha S, Shi R, Meng J. Behloul N, et al. Virus Res. 2020 Sep;286:198058. doi: 10.1016/j.virusres.2020.198058. Epub 2020 Jun 9. Virus Res. 2020. PMID: 32531235 Free PMC article. - Mass Spectrometry and Structural Biology Techniques in the Studies on the Coronavirus-Receptor Interaction.
Witkowska D. Witkowska D. Molecules. 2020 Sep 10;25(18):4133. doi: 10.3390/molecules25184133. Molecules. 2020. PMID: 32927621 Free PMC article. Review. - Unraveling the Epidemiology, Geographical Distribution, and Genomic Evolution of Potentially Lethal Coronaviruses (SARS, MERS, and SARS CoV-2).
Masood N, Malik SS, Raja MN, Mubarik S, Yu C. Masood N, et al. Front Cell Infect Microbiol. 2020 Aug 27;10:499. doi: 10.3389/fcimb.2020.00499. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32974224 Free PMC article. Review.
Cited by
- Immune Response, Inflammation, and the Clinical Spectrum of COVID-19.
García LF. García LF. Front Immunol. 2020 Jun 16;11:1441. doi: 10.3389/fimmu.2020.01441. eCollection 2020. Front Immunol. 2020. PMID: 32612615 Free PMC article. Review. - Role of cytotoxic T lymphocytes and interferon-γ in coronavirus infection: Lessons from murine coronavirus infections in mice.
Kyuwa S, Sugiura Y. Kyuwa S, et al. J Vet Med Sci. 2020 Oct 20;82(10):1410-1414. doi: 10.1292/jvms.20-0313. Epub 2020 Aug 5. J Vet Med Sci. 2020. PMID: 32759577 Free PMC article. Review. - Structural dynamics of SARS-CoV-2 variants: A health monitoring strategy for anticipating Covid-19 outbreaks.
Fantini J, Yahi N, Azzaz F, Chahinian H. Fantini J, et al. J Infect. 2021 Aug;83(2):197-206. doi: 10.1016/j.jinf.2021.06.001. Epub 2021 Jun 3. J Infect. 2021. PMID: 34089757 Free PMC article. - Thermostability, Tunability, and Tenacity of RNA as Rubbery Anionic Polymeric Materials in Nanotechnology and Nanomedicine-Specific Cancer Targeting with Undetectable Toxicity.
Binzel DW, Li X, Burns N, Khan E, Lee WJ, Chen LC, Ellipilli S, Miles W, Ho YS, Guo P. Binzel DW, et al. Chem Rev. 2021 Jul 14;121(13):7398-7467. doi: 10.1021/acs.chemrev.1c00009. Epub 2021 May 26. Chem Rev. 2021. PMID: 34038115 Free PMC article. Review. - How concerning is a SARS-CoV-2 variant of concern? Computational predictions and the variants labeling system.
Ashoor D, Marzouq M, Trabelsi K, Chlif S, Abotalib N, Khalaf NB, Ramadan AR, Fathallah MD. Ashoor D, et al. Front Cell Infect Microbiol. 2022 Aug 10;12:868205. doi: 10.3389/fcimb.2022.868205. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36034694 Free PMC article. Review.
References
- Lee N et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. New England Journal of Medicine 348, 1986–1994 (2003). - PubMed
References for Extended Data:
- Otwinowski Z & Minor W in Macromolecular Crystallography, Pt A Vol. 276 Methods in Enzymology 307–326 (1997). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI110700/AI/NIAID NIH HHS/United States
- P30 GM124165/GM/NIGMS NIH HHS/United States
- R35 GM118047/GM/NIGMS NIH HHS/United States
- S10 OD021527/OD/NIH HHS/United States
- R01 AI089728/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous