Two Functionally Distinct Serotonergic Projections into Hippocampus - PubMed (original) (raw)
Two Functionally Distinct Serotonergic Projections into Hippocampus
Alessandro Luchetti et al. J Neurosci. 2020.
Abstract
Hippocampus receives dense serotonergic input specifically from raphe nuclei. However, what information is carried by this input and its impact on behavior has not been fully elucidated. Here we used in vivo two-photon imaging of activity of hippocampal median raphe projection fibers in behaving male and female mice and identified two distinct populations: one linked to reward delivery and the other to locomotion. Local optogenetic manipulation of these fibers confirmed a functional role for these projections in the modulation of reward-induced behavior. The diverse function of serotonergic inputs suggests a key role in integrating locomotion and reward information into the hippocampal CA1.SIGNIFICANCE STATEMENT Information constantly flows in the hippocampus, but only some of it is captured as a memory. One potential process that discriminates which information should be remembered is concomitance with reward. In this work, we report a neuromodulatory pathway, which delivers reward signal as well as locomotion signal to the hippocampal CA1. We found that the serotonergic system delivers heterogeneous input that may be integrated by the hippocampus to support its mnemonic functions. It is dynamically involved in regulating behavior through interaction with the hippocampus. Our results suggest that the serotonergic system interacts with the hippocampus in a dynamic and behaviorally specific manner to regulate reward-related information processing.
Keywords: CA1; calcium imaging; in vivo; optogenetics; reward; serotonin.
Copyright © 2020 the authors.
Figures
Figure 1.
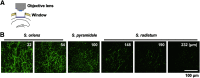
Distribution of serotonergic fibers in dorsal hippocampus. A, Imaging scheme. A part of overlaying cortex was removed, and an optical window was implanted, through which hippocampus could be observed under two-photon microscope. B, Serotonergic fibers in Sert-GFP transgenic mouse. The images were taken from the stratum oriens to the superficial portion of stratum radiatum using the same excitation and detection conditions. The numbers indicate depth from alveus.
Figure 2.
Imaging of serotonergic projections in CA1. A, Injection of AAV vector-expressing calcium indicator GCaMP6f into raphe. B, Representative histology of Sert-Cre mouse injected with floxed GCaMP6f virus. Diagram adapted from Paxinos and Franklin (2004). C, Schematic drawing of the VR used in this study. D, Representative example of animal's behavior in the virtual linear track and position (top), as well as speed and reward receipt (bottom). E, Representative images of serotonergic fiber in vivo (see Movie 1).
Figure 3.
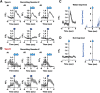
Dynamics of serotonergic fiber activity in CA1. A, B, Two populations of serotonergic fibers identified in hippocampal CA1 region. Type A: a reward-modulated fiber (A). Type B: locomotion-modulated fibers (B). Fluorescent intensity and running speed (blue) at reward delivery or run-start are shown. Example traces are shown with every individual reward and run-start instances taken from a single session (gray) and the average for that session (black) (A1,B1,A4,B4). The activity of the same fiber is shown at reward receipt and running start (one example fiber for Type A, one different example fiber for Type B). Averaged fluorescent activity (A2,B2,A5,B5) and running speed (A3,B3,A6,B6) of all fibers classified as Type A (n = 21 sessions from 3 animals) or Type B (n = 32 sessions from 4 animals) fibers are shown. Data were normalized by the baseline before the events (−3 to −1 s for run-start events and −2 −0 s for other events). A1, A4, Data were obtained from a single session from a mouse. The same applies to B1 and B4. C, D, Calcium activity at stopping of locomotion without receipt of reward (C, for Type A fibers, n = 8 sessions, 2 animals; D, for Type B fibers, n = 11 sessions, 3 animals). E, Calcium activity for Type B fibers averaged at instances of reward receipt that occurred without complete stop of locomotion (n = 12 sessions from 4 animals). F, Distribution of peak activity within times of reward receipt, run-start, immobility, or locomotion. For Type A fiber, reward frames had significantly more peak activity than other groups (for Type A fiber, repeated-measures one-way ANOVA, p < 0.05 or lower, n = 21 sessions from 3 animals). For Type B fiber, run-start frames had significantly more peak activity than other groups (repeated-measures one-way ANOVA, p < 0.05 or lower, n = 32 sessions from 4 animals). Data are mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; repeated-measures one-way ANOVA.
Figure 4.
Stability of the fiber activity and dependency to the reward. A, B, Representative trace of Type A and B fiber activity over consecutive days of imaging. The trace was averaged across events in the same day. C, Type A fiber activity to single unpredicted reward delivered in the absence of VR, randomly delivered while the animal is immobile. Black represents calcium activity. Blue represents speed. D, The same fiber but imaged 1 d later after the animal was given free access to water for 1 d and no longer thirsty. Data are mean ± SEM.
Figure 5.
Quantitative effects of multiple rewards on reward fiber dynamic. A, Type A fiber activity dynamic as animals collect more rewards within the same session. Pearson correlation, p = 0.0451 (n = 21 sessions from 3 animals). B, Averaged reward activity of a Type A fiber when 10 drops of reward (blue) or single-drop reward (red) were given. Repeated-measures two-way ANOVA (interaction between 1-drop and 10-drops groups significant after the peak, p = 0.0346, n = 11 reward events for 1-reward group, n = 4 reward events for 10-rewards group) from two different sessions in the same mouse. C, Quantitative assessment of fluorescence in the 0-2 and 2-9 s interval relative to a baseline for the 1-drop trace (p = 0.0327, paired t test, n = 11 events) and 10-drops trace (p = 0.0988, paired t test, n = 4 events). Data are mean ± SEM. *p < 0.05. n.s., not significant.
Figure 6.
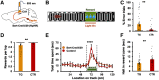
Inhibition of serotonergic fiber in CA1 modifies reward-induced behavior. A, B, Experimental settings. Optic cannulas were implanted bilaterally over dorsal hippocampal CA1 regions in transgenic mice expressing NpHR in serotonergic neurons. C, Percentage of errors during the performance of the virtual linear track task. Inhibition of CA1 serotonergic fibers significantly increased the number of errors (t test, p < 0.01, n = 34 sessions for transgenics [TG] and 23 sessions from controls [CTR], pooled from the 5 d duration of the task, from 7 transgenic mice and 5 control animals). D, Effect of the optogenetic inhibition on the reward collection performance. Inhibition of the serotonergic fibers in CA1 reduced the average number of rewards collected per lap (t test, p < 0.01, n = 34 sessions for transgenics and 23 sessions from controls, pooled from the 5 d duration of the task, from 7 transgenic mice and 5 control animals). E, Occupancy in the virtual linear track. Inhibition of serotonergic fibers in CA1 caused a reduction of time spent in the rewarded area (two-way repeated-measures ANOVA interaction, p < 0.01, post hoc test, p < 0.0001 for reward bin). F, Average halt per lap in reward zone. CA1 serotonergic fiber inhibition resulted in shorter halting in the reward zone compared with control animals (t test, p < 0.01, n = 34 sessions for transgenics and 23 sessions from controls, pooled from the 5 d duration of the task, from 7 transgenic mice and 5 control animals). Data are mean ± SEM. **p < 0.01, ****p < 0.0001.
Comment in
- Promoting Pleasant Memories with a Specialized Serotonergic Projection to the Hippocampus.
Giorgi A, Maddaloni G. Giorgi A, et al. J Neurosci. 2021 Jan 13;41(2):212-214. doi: 10.1523/JNEUROSCI.1615-20.2020. J Neurosci. 2021. PMID: 33441443 Free PMC article. No abstract available.
Similar articles
- Cell-Type-Specific Modulation of Sensory Responses in Olfactory Bulb Circuits by Serotonergic Projections from the Raphe Nuclei.
Brunert D, Tsuno Y, Rothermel M, Shipley MT, Wachowiak M. Brunert D, et al. J Neurosci. 2016 Jun 22;36(25):6820-35. doi: 10.1523/JNEUROSCI.3667-15.2016. J Neurosci. 2016. PMID: 27335411 Free PMC article. - Dedicated Hippocampal Inhibitory Networks for Locomotion and Immobility.
Arriaga M, Han EB. Arriaga M, et al. J Neurosci. 2017 Sep 20;37(38):9222-9238. doi: 10.1523/JNEUROSCI.1076-17.2017. Epub 2017 Aug 21. J Neurosci. 2017. PMID: 28842418 Free PMC article. - Hippocampal EEG and unit activity responses to modulation of serotonergic median raphe neurons in the freely behaving rat.
Nitz DA, McNaughton BL. Nitz DA, et al. Learn Mem. 1999 Mar-Apr;6(2):153-67. Learn Mem. 1999. PMID: 10327240 Free PMC article. - Serotonergic modulation of the limbic system.
Hensler JG. Hensler JG. Neurosci Biobehav Rev. 2006;30(2):203-14. doi: 10.1016/j.neubiorev.2005.06.007. Epub 2005 Sep 12. Neurosci Biobehav Rev. 2006. PMID: 16157378 Review.
Cited by
- Serotonin Signaling in Hippocampus during Initial Cocaine Abstinence Drives Persistent Drug Seeking.
Kohtz AS, Zhao J, Aston-Jones G. Kohtz AS, et al. J Neurosci. 2024 Apr 24;44(17):e1505212024. doi: 10.1523/JNEUROSCI.1505-21.2024. J Neurosci. 2024. PMID: 38514181 Free PMC article. - Neurometabolomic impacts of wood smoke and protective benefits of anti-aging therapeutics in aged female C57BL/6J mice.
Scieszka D, Hulse J, Gu H, Barkley-Levenson A, Barr E, Garcia M, Begay JG, Herbert G, McCormick M, Brigman J, Ottens A, Bleske B, Bhaskar K, Campen MJ. Scieszka D, et al. Res Sq [Preprint]. 2025 Mar 17:rs.3.rs-5936676. doi: 10.21203/rs.3.rs-5936676/v1. Res Sq. 2025. PMID: 40166007 Free PMC article. Preprint. - The role of the GABAergic cells of the median raphe region in reinforcement-based learning.
Chaves T, Török B, Fazekas C, Correia P, Karailiev P, Oravcova H, Sipos E, Biró L, Haller J, Jezova D, Zelena D. Chaves T, et al. Sci Rep. 2024 Jan 12;14(1):1175. doi: 10.1038/s41598-024-51743-y. Sci Rep. 2024. PMID: 38216718 Free PMC article. - Multiphoton imaging of hippocampal neural circuits: techniques and biological insights into region-, cell-type-, and pathway-specific functions.
Mizuta K, Sato M. Mizuta K, et al. Neurophotonics. 2024 Jul;11(3):033406. doi: 10.1117/1.NPh.11.3.033406. Epub 2024 Mar 8. Neurophotonics. 2024. PMID: 38464393 Free PMC article. Review. - Alternating sources of perisomatic inhibition during behavior.
Dudok B, Klein PM, Hwaun E, Lee BR, Yao Z, Fong O, Bowler JC, Terada S, Sparks FT, Szabo GG, Farrell JS, Berg J, Daigle TL, Tasic B, Dimidschstein J, Fishell G, Losonczy A, Zeng H, Soltesz I. Dudok B, et al. Neuron. 2021 Mar 17;109(6):997-1012.e9. doi: 10.1016/j.neuron.2021.01.003. Epub 2021 Feb 1. Neuron. 2021. PMID: 33529646 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous