Persistent Asymptomatic Human Infections by Salmonella enterica Serovar Newport in China - PubMed (original) (raw)
. 2020 May 27;5(3):e00163-20.
doi: 10.1128/mSphere.00163-20.
Hang Pan # 1, Beibei Wu # 3, Xiao Zhou 1, Xin Zhou 1, Wenqing Chai 1, Qingqing Wu 1, Shuning Li 1, Fang Li 1, Guimin Gu 4, Haoqiu Wang 5, Qinghua Hu 6, Xuebin Xu 7, Yan Li 1 8, Min Yue 9 8
Affiliations
- PMID: 32461269
- PMCID: PMC7253594
- DOI: 10.1128/mSphere.00163-20
Persistent Asymptomatic Human Infections by Salmonella enterica Serovar Newport in China
Narayan Paudyal et al. mSphere. 2020.
Abstract
Salmonella enterica serovar Newport (S Newport) infections are gradually on the rise in China from the last decade. For humans' infections, S Newport has been ranked among the top five serovars responsible for persistent infections, globally. A total of 290 S. Newport strains with their relevant clinical metadata were analyzed, and the strains were subjected to whole-genome sequence analysis. Among these, 62.4% (n = 181) were from diarrheic patients and 28.9% (n = 84) were from asymptomatic individuals (including adults and youngsters) while 8.6% (n = 25) were from cases of persistent diarrhea in infants (28%, n = 7) and toddlers (72%, n = 18). The association between the sequence types (STs) and the variations in the clinical presentation was statistically significant (P = 0.0432), with ST46 causing diarrhea or representing asymptomatic patients and ST31 or ST68 causing persistent diarrhea. Genomic analysis revealed that the highest proportion of the isolates (98.5%, n = 279), primarily from patients with or without diarrhea rather than from asymptomatic individuals, carried antimicrobial resistance determinants corresponding to the aminoglycosides and beta-lactams, highlighting the need for cautionary usage of antimicrobials in such patients. These findings also suggest that cases of nontyphoidal Salmonella infection with symptoms of acute diarrhea or persistent diarrhea caused by S Newport should be handled with caution, due to the high chance of development of an antimicrobial resistance phenotype that might lead to therapeutic failures. Together, S Newport ST31 and ST46, which have the highest frequency of carriage of multidrug resistance, are potentially responsible for antimicrobial-resistant diarrhea/persistent diarrhea in infants and children, while adult humans are more likely to be (asymptomatic) carriers of the S Newport strains.IMPORTANCE Human infections caused by Salmonella Newport generally lead to gastrointestinal diseases. These infections are normally self-limiting; however, in certain cases, broad-spectrum antimicrobials are prescribed for the treatment. The Chinese National Foodborne Disease Surveillance Network has reported a gradual increase in the incidence of multidrug-resistant S Newport infections in humans. After careful evaluation of the dynamic relationship among the clinical findings, the age group, and the genomic sequence data, it was found that young patients represented the major group with persistent diarrhea, whereas adults were either asymptomatic or diarrheic. Furthermore, all these strains contained multiple acquired antimicrobial resistance determinants, which limited the use of antimicrobials for human patients of all age groups. This analysis of the laboratory-confirmed cases, coupled with genetic analysis of the corresponding pathogen, revealed that antimicrobial treatment of persistent infections by S Newport in infants and toddlers, and in asymptomatic or diarrheic adults, may not be successful. If the antimicrobials must be prescribed at all, they must be used with caution because of the presence of multiple acquired antimicrobial resistance determinants in such strains.
Keywords: Salmonella Newport; age; antimicrobial resistance; human symptoms.
Copyright © 2020 Paudyal et al.
Figures
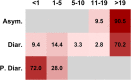
FIG 1
Prevalence by age, symptoms, and clinical findings. Data represent percentages of S. Newport (values of individual cells) isolated from different clinical presentations (left side) for various age categories (patient age on top). Blank cells represent the absence of data. The age categories (x axis, in years, top) are classified as infant (<1 year of age), toddler (1 to 5 years of age), child (6 to 10 years of age), adolescent (11 to 19 years of age), and adult (>19 years of age). The symptoms (y axis) are abbreviated as follows: asymptomatic, Asym.; diarrhea, Diar.; persistent diarrhea, P. Diar.
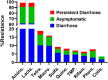
FIG 2
Antimicrobial resistance and clinical findings. Data represent percentages of carriage of acquired resistance genetic determinants (genes) by the S. Newport strains isolated from various clinical presentations. The x axis gives the classes of antimicrobials as follows: Amino., aminoglycosides; Lacta., beta-lactams; Tetra., tetracyclines; Macro., macrolides; Sulfa., sulfonamides; Quino., quinolones; TMP, trimethoprim; Rifam., rifampin; Pheni., phenicol; Colis., colistin.
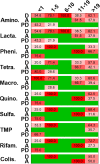
FIG 3
Antimicrobial resistance, age, and clinical findings. The heat map shows the percentages of isolates that were genotypically resistant for the given category of drugs (as described in the Fig. 2 legend) and the clinical symptoms (D, diarrhea; A, asymptomatic; PD, persistent diarrhea) as indicated on the right. The top gives the classifications of age (as described in the Fig. 1 legend). The shades of red show the various percentages of carriage of acquired resistance determinants, while green indicates that the isolates did not contain any such determinants, based on genomic analysis. Values in the cells indicate percentages of carriage of acquired antimicrobial resistance determinants.
Similar articles
- Serovar distribution, antimicrobial resistance profiles, and PFGE typing of Salmonella enterica strains isolated from 2007-2012 in Guangdong, China.
Ke B, Sun J, He D, Li X, Liang Z, Ke CW. Ke B, et al. BMC Infect Dis. 2014 Jun 17;14:338. doi: 10.1186/1471-2334-14-338. BMC Infect Dis. 2014. PMID: 24939394 Free PMC article. - Genomic Epidemiology of Multidrug-Resistant Nontyphoidal Salmonella in Young Children Hospitalized for Gastroenteritis.
Woh PY, Yeung MPS, Goggins WB 3rd, Lo N, Wong KT, Chow V, Chau KY, Fung K, Chen Z, Ip M. Woh PY, et al. Microbiol Spectr. 2021 Sep 3;9(1):e0024821. doi: 10.1128/Spectrum.00248-21. Epub 2021 Aug 4. Microbiol Spectr. 2021. PMID: 34346743 Free PMC article. - Genomic investigation of Salmonella enterica Serovar Welikade from a pediatric diarrhea case first time in Shanghai, China.
Shen Y, Zhou Y, Gong J, Li G, Liu Y, Xu X, Chen M. Shen Y, et al. BMC Genomics. 2024 Jun 17;25(1):604. doi: 10.1186/s12864-024-10489-7. BMC Genomics. 2024. PMID: 38886668 Free PMC article. - Prevalence and multidrug resistance in Salmonella enterica Typhimurium: an overview in South East Asia.
Patra SD, Mohakud NK, Panda RK, Sahu BR, Suar M. Patra SD, et al. World J Microbiol Biotechnol. 2021 Sep 28;37(11):185. doi: 10.1007/s11274-021-03146-8. World J Microbiol Biotechnol. 2021. PMID: 34580741 Review. - Epidemiology and Genomics of Invasive Nontyphoidal Salmonella Infections in Kenya.
Kariuki S, Onsare RS. Kariuki S, et al. Clin Infect Dis. 2015 Nov 1;61 Suppl 4(Suppl 4):S317-24. doi: 10.1093/cid/civ711. Clin Infect Dis. 2015. PMID: 26449947 Free PMC article. Review.
Cited by
- Antimicrobial resistance and genomic investigation of non-typhoidal Salmonella isolated from outpatients in Shaoxing city, China.
Chen J, Ed-Dra A, Zhou H, Wu B, Zhang Y, Yue M. Chen J, et al. Front Public Health. 2022 Sep 13;10:988317. doi: 10.3389/fpubh.2022.988317. eCollection 2022. Front Public Health. 2022. PMID: 36176509 Free PMC article. - Genomic Determinants of Pathogenicity and Antimicrobial Resistance for 60 Global Listeria monocytogenes Isolates Responsible for Invasive Infections.
Shi D, Anwar TM, Pan H, Chai W, Xu S, Yue M. Shi D, et al. Front Cell Infect Microbiol. 2021 Oct 27;11:718840. doi: 10.3389/fcimb.2021.718840. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34778102 Free PMC article. - Genomic characterization of Salmonella Uzaramo for human invasive infection.
Xu X, Chen Y, Pan H, Pang Z, Li F, Peng X, Ed-Dra A, Li Y, Yue M. Xu X, et al. Microb Genom. 2020 Jul;6(7):mgen000401. doi: 10.1099/mgen.0.000401. Microb Genom. 2020. PMID: 32589568 Free PMC article. - Lacticaseibacillus rhamnosus alleviates intestinal inflammation and promotes microbiota-mediated protection against Salmonella fatal infections.
Peng X, Ed-Dra A, Song Y, Elbediwi M, Nambiar RB, Zhou X, Yue M. Peng X, et al. Front Immunol. 2022 Aug 11;13:973224. doi: 10.3389/fimmu.2022.973224. eCollection 2022. Front Immunol. 2022. PMID: 36032095 Free PMC article. - Characterization of Salmonella serotypes prevalent in asymptomatic people and patients.
Xu H, Zhang W, Zhang K, Zhang Y, Wang Z, Zhang W, Li Y, Li Q. Xu H, et al. BMC Infect Dis. 2021 Jul 1;21(1):632. doi: 10.1186/s12879-021-06340-z. BMC Infect Dis. 2021. PMID: 34210275 Free PMC article.
References
- GBD 2016 Diarrhoeal Disease Collaborators. 2018. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 18:1211–1228. doi:10.1016/S1473-3099(18)30362-1. - DOI - PMC - PubMed
- Wang X, Biswas S, Paudyal N, Pan H, Li X, Fang W, Yue M. 2019. Antimicrobial resistance in Salmonella typhimurium isolates recovered from the food chain through national antimicrobial resistance monitoring system between 1996 and 2016. Front Microbiol 10:985. doi:10.3389/fmicb.2019.00985. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical