LOMIX, a Mixture of Flaxseed Linusorbs, Exerts Anti-Inflammatory Effects through Src and Syk in the NF-κB Pathway - PubMed (original) (raw)
LOMIX, a Mixture of Flaxseed Linusorbs, Exerts Anti-Inflammatory Effects through Src and Syk in the NF-κB Pathway
Zubair Ahmed Ratan et al. Biomolecules. 2020.
Abstract
Although flax (Linum usitatissimum L.) has long been used as Ayurvedic medicine, its anti-inflammatory role is still unclear. Therefore, we aimed to investigate the anti-inflammatory role of a linusorb mixture (LOMIX) recovered from flaxseed oil. Effects of LOMIX on inflammation and its mechanism of action were examined using several in vitro assays (i.e., NO production, real-time PCR analysis, luciferase-reporter assay, Western blot analysis, and kinase assay) and in vivo analysis with animal inflammation models as well as acute toxicity test. Results: LOMIX inhibited NO production, cell shape change, and inflammatory gene expression in stimulated RAW264.7 cells through direct targeting of Src and Syk in the NF-κB pathway. In vivo study further showed that LOMIX alleviated symptoms of gastritis, colitis, and hepatitis in murine model systems. In accordance with in vitro results, the in vivo anti-inflammatory effects were mediated by inhibition of Src and Syk. LOMIX was neither cytotoxic nor did it cause acute toxicity in mice. In addition, it was found that LOB3, LOB2, and LOA2 are active components included in LOMIX, as assessed by NO assay. These in vitro and in vivo results suggest that LOMIX exerts an anti-inflammatory effect by inhibiting the inflammatory responses of macrophages and ameliorating symptoms of inflammatory diseases without acute toxicity and is a promising anti-inflammatory medication for inflammatory diseases.
Keywords: LOMIX; NF-κB; Src; Syk; anti-inflammatory; flaxseed oil; linusorb.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
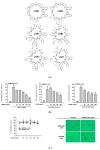
Figure 1
Linusorb mixture (LOMIX) has anti-inflammatory activity in macrophages. (A) Chemical structures of LOMIX components. (B) RAW264.7 cells pretreated with the indicated doses of LOMIX for 30 min were treated with either LPS (1 µg/mL), Pam3CSK4 (10 µg/mL), or Poly(I:C) (200 µg/mL) for 24 h. The NO level in cell culture media was determined by Griess assay. (C) RAW264.7 and HEK293 cells were treated with the indicated doses of LOMIX for 24 h, and the cell viability was determined by MTT assay (left panel). Photos were taken with a digital camera (right panel). (D) RAW264.7 cells pretreated with the indicated doses of either (D left) L-NAME or (D middle) Pred for 30 min were treated with LPS (1 µg/mL) for 24 h, and NO level in the cell culture media was determined by Griess assay. (D right) RAW264.7 cells were treated with the indicated doses of either L-NAME or Pred for 24 h, and the cell viability was determined by MTT assay. RAW264.7 cells pretreated with LOMIX (200 µg/mL) for 30 min were treated with LPS (1 µg/mL) for 24 h. (E left) Cell shape was photographed, and (E right) the number of cells with an altered shape was counted using a hemocytometer and plotted. ## p < 0.01 compared to the vehicle-treated control. * p < 0.05, ** p < 0.01 compared to the stimulator-treated controls. Original magnification of (E) was 250×.
Figure 1
Linusorb mixture (LOMIX) has anti-inflammatory activity in macrophages. (A) Chemical structures of LOMIX components. (B) RAW264.7 cells pretreated with the indicated doses of LOMIX for 30 min were treated with either LPS (1 µg/mL), Pam3CSK4 (10 µg/mL), or Poly(I:C) (200 µg/mL) for 24 h. The NO level in cell culture media was determined by Griess assay. (C) RAW264.7 and HEK293 cells were treated with the indicated doses of LOMIX for 24 h, and the cell viability was determined by MTT assay (left panel). Photos were taken with a digital camera (right panel). (D) RAW264.7 cells pretreated with the indicated doses of either (D left) L-NAME or (D middle) Pred for 30 min were treated with LPS (1 µg/mL) for 24 h, and NO level in the cell culture media was determined by Griess assay. (D right) RAW264.7 cells were treated with the indicated doses of either L-NAME or Pred for 24 h, and the cell viability was determined by MTT assay. RAW264.7 cells pretreated with LOMIX (200 µg/mL) for 30 min were treated with LPS (1 µg/mL) for 24 h. (E left) Cell shape was photographed, and (E right) the number of cells with an altered shape was counted using a hemocytometer and plotted. ## p < 0.01 compared to the vehicle-treated control. * p < 0.05, ** p < 0.01 compared to the stimulator-treated controls. Original magnification of (E) was 250×.
Figure 2
LOMIX suppresses mRNA expression of inflammatory genes by inhibiting NF-κB. RAW264.7 cells pretreated with the indicated doses of LOMIX for 30 min were treated with LPS (1 µg/mL) for 6 h, and mRNA expression levels of iNOS, COX-2, and TNF-α were determined by (A) quantitative real-time PCR and (B) semiquantitative RT-PCR. (C) HEK293 cells pretreated with the indicated doses of LOMIX for 24 h were transfected with the indicated plasmids for another 24 h. NF-κB luciferase reporter gene activity was determined by luminometer and normalized to that of β-galactosidase. (D) RAW264.7 cells pretreated with LOMIX (200 µg/mL) for 30 min were treated with LPS (1 µg/mL) for the indicated time. p65 and p50 in nuclear lysates were detected by Western blot analysis, and lamin A/C was used as an internal control. ## p < 0.01 compared to the vehicle-treated control. ** p < 0.01 compared to the stimulator-treated controls.
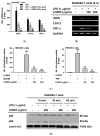
Figure 3
LOMIX suppresses NF-κB signaling. RAW264.7 cells pretreated with LOMIX (200 µg/mL) for 30 min were treated with LPS (1 µg/mL) for the indicated time. (A) p-IκBα and IκBα in whole cell lysates were detected by Western blot analysis. (B left) RAW264.7 cells pretreated with LOMIX (200 µg/mL) for 30 min were treated with LPS (1 µg/mL) for the indicated time, and p-Src, Src, p-Syk, Syk, p-p85, and p85 in whole cell lysates were detected by Western blot analysis. (B right) RAW264.7 cells pretreated with the indicated doses of LOMIX for 30 min were treated with LPS (1 µg/mL) for 5 min, and p-Src, Src, p-Syk, and Syk in whole cell lysates were detected by Western blot analysis. (C) HEK293 cells pretreated with the indicated doses of LOMIX for 24 h were transfected with the indicated plasmids for another 24 h, and p-Src, Src, p-Syk, Syk, and hemagglutinin (HA) in whole cell lysates were detected by Western blot analysis. β-actin and lamin A/C were used as internal controls for whole cell and nuclear lysates, respectively. (D) Inhibitory activity of LOMIX on Src and Syk kinase activities was determined by an in vitro kinase assay with purified enzymes. ** p < 0.01 compared to the vehicle-treated control.
Figure 3
LOMIX suppresses NF-κB signaling. RAW264.7 cells pretreated with LOMIX (200 µg/mL) for 30 min were treated with LPS (1 µg/mL) for the indicated time. (A) p-IκBα and IκBα in whole cell lysates were detected by Western blot analysis. (B left) RAW264.7 cells pretreated with LOMIX (200 µg/mL) for 30 min were treated with LPS (1 µg/mL) for the indicated time, and p-Src, Src, p-Syk, Syk, p-p85, and p85 in whole cell lysates were detected by Western blot analysis. (B right) RAW264.7 cells pretreated with the indicated doses of LOMIX for 30 min were treated with LPS (1 µg/mL) for 5 min, and p-Src, Src, p-Syk, and Syk in whole cell lysates were detected by Western blot analysis. (C) HEK293 cells pretreated with the indicated doses of LOMIX for 24 h were transfected with the indicated plasmids for another 24 h, and p-Src, Src, p-Syk, Syk, and hemagglutinin (HA) in whole cell lysates were detected by Western blot analysis. β-actin and lamin A/C were used as internal controls for whole cell and nuclear lysates, respectively. (D) Inhibitory activity of LOMIX on Src and Syk kinase activities was determined by an in vitro kinase assay with purified enzymes. ** p < 0.01 compared to the vehicle-treated control.
Figure 4
LOMIX ameliorates the symptoms of inflammatory diseases in mice. (A) ICR mice received the indicated doses of LOMIX or ranitidine (40 mg/kg) orally twice a day for 3 days. Thirty minutes after the final administration, experimental gastritis was induced by administering HCl/EtOH to the mice for 1 h. (A left) Ulcerative gastritis lesions were photographed, and (A middle) the areas of gastritis lesions were measured using ImageJ software and plotted. (A right) p-Src, Src, p-Syk, Syk, and p-p65 in whole stomach lysates were detected by Western blot analysis. (B) ICR mice received the indicated doses of LOMIX orally twice a day for 3 days. Thirty minutes after the final administration, experimental colitis was induced by administering 3% DSS (v/v) to the mice for 7 days. (B left) Colons were photographed, and (B middle) colon length was measured using calipers and plotted. (B right) Colon tissue was stained with H&E for histological analysis. (C) ICR mice received the indicated doses of LOMIX orally twice a day for 3 days. Thirty minutes after the final administration, experimental hepatitis was induced by administering LPS (10 µg/kg) and D-GalN (1 mg/kg) to the mice for 6 h. (C left) Liver tissues were subjected to H&E staining for histological analysis. (C middle) Serum levels of AST and ALT were determined by ELISA. (C right) p-Src and p-p65 in whole liver lysates were detected by Western blot analysis. β-actin was used as an internal control. ## p < 0.01 compared to the vehicle-treated control. * p < 0.05, ** p < 0.01 compared to the stimulator-treated controls.
Figure 5
In vivo acute toxicity study of LOMIX. ICR mice received a single dose of LOMIX (5 g/kg) orally, and organs (brain, thymus, heart, lung, liver, spleen, stomach, kidney, bladder, and colon) were excised after 24 h. (A) Organs were photographed, and (B) the weight of the organs was measured using a scale. (C) Caspase-3 and Bcl-2 in whole lysates of each organ were detected by Western blot analysis, and β-actin was used as an internal control.
Figure 5
In vivo acute toxicity study of LOMIX. ICR mice received a single dose of LOMIX (5 g/kg) orally, and organs (brain, thymus, heart, lung, liver, spleen, stomach, kidney, bladder, and colon) were excised after 24 h. (A) Organs were photographed, and (B) the weight of the organs was measured using a scale. (C) Caspase-3 and Bcl-2 in whole lysates of each organ were detected by Western blot analysis, and β-actin was used as an internal control.
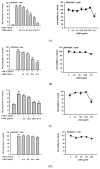
Figure 6
Anti-inflammatory effect of each linusorb in LOMIX. RAW264.7 cells pretreated with the indicated doses of either (A left) LOB3, (B left) LOB2, (C left) LOA2, (D left) LOB1, or (E left) LOA1/A3 for 30 min were treated with LPS (1 µg/mL) for 24 h, and NO level in the cell culture media was determined by Griess assay. RAW264.7 cells were treated with the indicated doses of either (A right) LOB3, (B right) LOB2, (C right) LOA2, (D right) LOB1, or (E right) LOA1/A3 for 24 h, and the cell viability was determined by MTT assay. (F) IC50 values of LOB3, LOB2, LOA2, and LOA1/A3 for NO production. ## p < 0.01 compared to the vehicle-treated control. * p < 0.05, ** p < 0.01 compared to the stimulator-treated controls.
Figure 6
Anti-inflammatory effect of each linusorb in LOMIX. RAW264.7 cells pretreated with the indicated doses of either (A left) LOB3, (B left) LOB2, (C left) LOA2, (D left) LOB1, or (E left) LOA1/A3 for 30 min were treated with LPS (1 µg/mL) for 24 h, and NO level in the cell culture media was determined by Griess assay. RAW264.7 cells were treated with the indicated doses of either (A right) LOB3, (B right) LOB2, (C right) LOA2, (D right) LOB1, or (E right) LOA1/A3 for 24 h, and the cell viability was determined by MTT assay. (F) IC50 values of LOB3, LOB2, LOA2, and LOA1/A3 for NO production. ## p < 0.01 compared to the vehicle-treated control. * p < 0.05, ** p < 0.01 compared to the stimulator-treated controls.
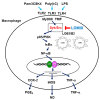
Figure 7
Schematic summary describing LOMIX-mediated anti-inflammatory activity in macrophages.
Similar articles
- Ranunculus bulumei Methanol Extract Exerts Anti-Inflammatory Activity by Targeting Src/Syk in NF-κB Signaling.
Hong YH, Kim JH, Cho JY. Hong YH, et al. Biomolecules. 2020 Apr 3;10(4):546. doi: 10.3390/biom10040546. Biomolecules. 2020. PMID: 32260181 Free PMC article. - Anti-inflammatory activities of Canarium subulatum Guillaumin methanol extract operate by targeting Src and Syk in the NF-κB pathway.
Choi E, Kim MY, Cho JY. Choi E, et al. J Ethnopharmacol. 2019 Jun 28;238:111848. doi: 10.1016/j.jep.2019.111848. Epub 2019 Apr 2. J Ethnopharmacol. 2019. PMID: 30951845 - A comprehensive review of flaxseed (Linum usitatissimum L.): health-affecting compounds, mechanism of toxicity, detoxification, anticancer and potential risk.
Mueed A, Shibli S, Jahangir M, Jabbar S, Deng Z. Mueed A, et al. Crit Rev Food Sci Nutr. 2023;63(32):11081-11104. doi: 10.1080/10408398.2022.2092718. Epub 2022 Jul 14. Crit Rev Food Sci Nutr. 2023. PMID: 35833457 Review. - Utilizing the healing power of flaxseed: a narrative review of its therapeutic applications.
Mohammadkhani N, Moradnia M, Mohammadi M, Ebrahimpour S, Tabatabaei-Malazy O, Mirsadeghi S, Javar HA. Mohammadkhani N, et al. J Diabetes Metab Disord. 2025 May 28;24(1):128. doi: 10.1007/s40200-025-01636-2. eCollection 2025 Jun. J Diabetes Metab Disord. 2025. PMID: 40454185 Review.
Cited by
- The Anti-Inflammatory Mechanism of Flaxseed Linusorbs on Lipopolysaccharide-Induced RAW 264.7 Macrophages by Modulating TLR4/NF-κB/MAPK Pathway.
Li J, Chen J, Huang P, Cai Z, Zhang N, Wang Y, Li Y. Li J, et al. Foods. 2023 Jun 16;12(12):2398. doi: 10.3390/foods12122398. Foods. 2023. PMID: 37372610 Free PMC article. - The Role of Flaxseed in Improving Human Health.
Nowak W, Jeziorek M. Nowak W, et al. Healthcare (Basel). 2023 Jan 30;11(3):395. doi: 10.3390/healthcare11030395. Healthcare (Basel). 2023. PMID: 36766971 Free PMC article. Review. - The Anti-Cancer Effect of Linusorb B3 from Flaxseed Oil through the Promotion of Apoptosis, Inhibition of Actin Polymerization, and Suppression of Src Activity in Glioblastoma Cells.
Sung NY, Jeong D, Shim YY, Ratan ZA, Jang YJ, Reaney MJT, Lee S, Lee BH, Kim JH, Yi YS, Cho JY. Sung NY, et al. Molecules. 2020 Dec 12;25(24):5881. doi: 10.3390/molecules25245881. Molecules. 2020. PMID: 33322712 Free PMC article. - Uptake of Flaxseed Dietary Linusorbs Modulates Regulatory Genes Including Induction of Heat Shock Proteins and Apoptosis.
Shim YY, Tse TJ, Saini AK, Kim YJ, Reaney MJT. Shim YY, et al. Foods. 2022 Nov 22;11(23):3761. doi: 10.3390/foods11233761. Foods. 2022. PMID: 36496568 Free PMC article. - Anti-Melanogenesis Effects of a Cyclic Peptide Derived from Flaxseed via Inhibition of CREB Pathway.
Yoon JH, Jang WY, Park SH, Kim HG, Shim YY, Reaney MJT, Cho JY. Yoon JH, et al. Int J Mol Sci. 2022 Dec 28;24(1):536. doi: 10.3390/ijms24010536. Int J Mol Sci. 2022. PMID: 36613979 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous