Identification of the PANoptosome: A Molecular Platform Triggering Pyroptosis, Apoptosis, and Necroptosis (PANoptosis) - PubMed (original) (raw)
doi: 10.3389/fcimb.2020.00237. eCollection 2020.
Min Zheng 1, Sannula Kesavardhana 1, Rajendra Karki 1, R K Subbarao Malireddi 1, Balaji Banoth 1, David E Place 1, Benoit Briard 1, Bhesh Raj Sharma 1, Shraddha Tuladhar 1, Parimal Samir 1, Amanda Burton 1, Thirumala-Devi Kanneganti 1
Affiliations
- PMID: 32547960
- PMCID: PMC7274033
- DOI: 10.3389/fcimb.2020.00237
Identification of the PANoptosome: A Molecular Platform Triggering Pyroptosis, Apoptosis, and Necroptosis (PANoptosis)
Shelbi Christgen et al. Front Cell Infect Microbiol. 2020.
Abstract
Programmed cell death plays crucial roles in organismal development and host defense. Recent studies have highlighted mechanistic overlaps and extensive, multifaceted crosstalk between pyroptosis, apoptosis, and necroptosis, three programmed cell death pathways traditionally considered autonomous. The growing body of evidence, in conjunction with the identification of molecules controlling the concomitant activation of all three pathways by pathological triggers, has led to the development of the concept of PANoptosis. During PANoptosis, inflammatory cell death occurs through the collective activation of pyroptosis, apoptosis, and necroptosis, which can circumvent pathogen-mediated inhibition of individual death pathways. Many of the molecular details of this emerging pathway are unclear. Here, we describe the activation of PANoptosis by bacterial and viral triggers and report protein interactions that reveal the formation of a PANoptosome complex. Infection of macrophages with influenza A virus, vesicular stomatitis virus, Listeria monocytogenes, or Salmonella enterica serovar Typhimurium resulted in robust cell death and the hallmarks of PANoptosis activation. Combined deletion of the PANoptotic components caspase-1 (CASP1), CASP11, receptor-interacting serine/threonine-protein kinase 3 (RIPK3), and CASP8 largely protected macrophages from cell death induced by these pathogens, while deletion of individual components provided reduced or no protection. Further, molecules from the pyroptotic, apoptotic, and necroptotic cell death pathways interacted to form a single molecular complex that we have termed the PANoptosome. Overall, our study identifies pathogens capable of activating PANoptosis and the formation of a PANoptosome complex.
Keywords: ASC; NLRP3; PANoptosis; PANoptosome; RIPK1; RIPK3; caspase-1; caspase-8.
Copyright © 2020 Christgen, Zheng, Kesavardhana, Karki, Malireddi, Banoth, Place, Briard, Sharma, Tuladhar, Samir, Burton and Kanneganti.
Figures
Figure 1
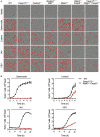
Loss of PANoptotic molecules prevents infection-induced cell death. Cell death analysis of BMDMs lacking different components of pyroptosis, apoptosis, or necroptosis. (A) Representative cell death images with the red mask indicating dead cells and (B) quantification of cell death over time in BMDMs after S. Typhimurium, L. monocytogenes, IAV, and VSV infection.
Figure 2
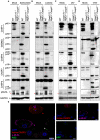
Bacterial and viral infections activate PANoptosis in vitro. Western blot analysis of PANoptosis activation markers after (A) S. Typhimurium, (B) L. monocytogenes, (C) IAV, and (D) VSV infection. Activation of pyroptosis was measured by immunoblotting of cleaved CASP1 (p20) and activated GSDMD (p20/p30). Activation of apoptosis was measured by immunoblotting of active CASP8 (p18), active CASP7 (p20), and active CASP3 (p17/19). Activation of necroptosis was measured by phosphorylation of MLKL (pMLKL). Red asterisks denote a non-specific band. (E) Confocal imaging of PANoptosis activation in wild-type BMDMs following S. Typhimurium infection. ASC speck formation (white triangle), cleavage of CASP3 (red triangle), and pMLKL localization to the membrane (purple triangle) were used as readouts of PANoptosis. Scale bar, 10 μm.
Figure 3
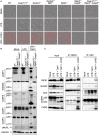
PANoptotic molecules directly interact. (A) Co-immunoprecipitation of NLRP3 expressed in HEK293T cells with ZBP1, RIPK3, and RIPK1 individually. (B) Co-immunoprecipitation of the PANoptosome complex in HEK293T cells expressing CASP8, ASC, RIPK1, NLRP3, and ZBP1 with or without RIPK3. Red asterisks denote a non-specific band.
Figure 4
Inhibition of TAK1 promotes PANoptosis and PANoptosome formation. (A) Representative cell death images of BMDMs lacking different components of pyroptosis, apoptosis, or necroptosis after LPS priming and inhibition of TAK1. (B) Western blot analysis of PANoptosis activation after LPS priming and TAK1 inhibition. (C) Co-immunoprecipitation of PANoptosome components from primary BMDMs after TAK1 and caspase inhibition. Red asterisks denote a non-specific band.
Figure 5
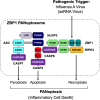
Graphical representation of a PANoptosome. Schematic summary of a representative PANoptosome formed after IAV infection. Domains hypothesized to be crucial for mediating PANoptosome formation are highlighted.
Similar articles
- From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways.
Wang Y, Kanneganti TD. Wang Y, et al. Comput Struct Biotechnol J. 2021 Aug 3;19:4641-4657. doi: 10.1016/j.csbj.2021.07.038. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34504660 Free PMC article. Review. - Single cell analysis of PANoptosome cell death complexes through an expansion microscopy method.
Wang Y, Pandian N, Han JH, Sundaram B, Lee S, Karki R, Guy CS, Kanneganti TD. Wang Y, et al. Cell Mol Life Sci. 2022 Sep 28;79(10):531. doi: 10.1007/s00018-022-04564-z. Cell Mol Life Sci. 2022. PMID: 36169732 Free PMC article. - The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis).
Samir P, Malireddi RKS, Kanneganti TD. Samir P, et al. Front Cell Infect Microbiol. 2020 Jun 3;10:238. doi: 10.3389/fcimb.2020.00238. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32582562 Free PMC article. Review. - RIPK1 Distinctly Regulates _Yersinia_-Induced Inflammatory Cell Death, PANoptosis.
Malireddi RKS, Kesavardhana S, Karki R, Kancharana B, Burton AR, Kanneganti TD. Malireddi RKS, et al. Immunohorizons. 2020 Dec 11;4(12):789-796. doi: 10.4049/immunohorizons.2000097. Immunohorizons. 2020. PMID: 33310881 Free PMC article. - Immune regulator IRF1 contributes to ZBP1-, AIM2-, RIPK1-, and NLRP12-PANoptosome activation and inflammatory cell death (PANoptosis).
Sharma BR, Karki R, Rajesh Y, Kanneganti TD. Sharma BR, et al. J Biol Chem. 2023 Sep;299(9):105141. doi: 10.1016/j.jbc.2023.105141. Epub 2023 Aug 7. J Biol Chem. 2023. PMID: 37557956 Free PMC article.
Cited by
- Pyroptosis regulation by Salmonella effectors.
Meng Y, Zhang Q, Xu M, Ding K, Yu Z, Li J. Meng Y, et al. Front Immunol. 2024 Oct 23;15:1464858. doi: 10.3389/fimmu.2024.1464858. eCollection 2024. Front Immunol. 2024. PMID: 39507539 Free PMC article. Review. - The assembly and activation of the PANoptosome promote porcine granulosa cell programmed cell death during follicular atresia.
Wu H, Han Y, Liu J, Zhao R, Dai S, Guo Y, Li N, Yang F, Zeng S. Wu H, et al. J Anim Sci Biotechnol. 2024 Nov 5;15(1):147. doi: 10.1186/s40104-024-01107-3. J Anim Sci Biotechnol. 2024. PMID: 39497227 Free PMC article. - Advancements in programmed cell death research in antitumor therapy: a comprehensive overview.
Wei S, Han C, Mo S, Huang H, Luo X. Wei S, et al. Apoptosis. 2024 Nov 2. doi: 10.1007/s10495-024-02038-0. Online ahead of print. Apoptosis. 2024. PMID: 39487314 Review. - Mechanism of lactic acidemia-promoted pulmonary endothelial cells death in sepsis: role for CIRP-ZBP1-PANoptosis pathway.
Gong T, Wang QD, Loughran PA, Li YH, Scott MJ, Billiar TR, Liu YT, Fan J. Gong T, et al. Mil Med Res. 2024 Oct 28;11(1):71. doi: 10.1186/s40779-024-00574-z. Mil Med Res. 2024. PMID: 39465383 Free PMC article. - Inflammasome protein scaffolds the DNA damage complex during tumor development.
Shen C, Pandey A, Enosi Tuipulotu D, Mathur A, Liu L, Yang H, Adikari NK, Ngo C, Jing W, Feng S, Hao Y, Zhao A, Kirkby M, Kurera M, Zhang J, Venkataraman S, Liu C, Song R, Wong JJ, Schumann U, Natoli R, Wen J, Zhang L, Kaakoush NO, Man SM. Shen C, et al. Nat Immunol. 2024 Nov;25(11):2085-2096. doi: 10.1038/s41590-024-01988-6. Epub 2024 Oct 14. Nat Immunol. 2024. PMID: 39402152
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI101935/AI/NIAID NIH HHS/United States
- R01 AI124346/AI/NIAID NIH HHS/United States
- R01 CA163507/CA/NCI NIH HHS/United States
- R37 AI101935/AI/NIAID NIH HHS/United States
- R01 AR056296/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous