Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study - PubMed (original) (raw)
Clinical Trial
. 2020 Aug;7(8):e575-e582.
doi: 10.1016/S2352-3026(20)30216-7. Epub 2020 Jun 30.
Alexander B Pine 1, Matthew L Meizlish 2, C-Hong Chang 3, Hanming Zhang 3, Parveen Bahel 4, Audrey Baluha 5, Noffar Bar 1, Robert D Bona 1, Adrienne J Burns 5, Charles S Dela Cruz 6, Anne Dumont 5, Stephanie Halene 1, John Hwa 3, Jonathan Koff 6, Hope Menninger 5, Natalia Neparidze 1, Christina Price 7, Jonathan M Siner 6, Christopher Tormey 4, Henry M Rinder 4, Hyung J Chun 3, Alfred I Lee 8
Affiliations
- PMID: 32619411
- PMCID: PMC7326446
- DOI: 10.1016/S2352-3026(20)30216-7
Clinical Trial
Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study
George Goshua et al. Lancet Haematol. 2020 Aug.
Abstract
Background: An important feature of severe acute respiratory syndrome coronavirus 2 pathogenesis is COVID-19-associated coagulopathy, characterised by increased thrombotic and microvascular complications. Previous studies have suggested a role for endothelial cell injury in COVID-19-associated coagulopathy. To determine whether endotheliopathy is involved in COVID-19-associated coagulopathy pathogenesis, we assessed markers of endothelial cell and platelet activation in critically and non-critically ill patients admitted to the hospital with COVID-19.
Methods: In this single-centre cross-sectional study, hospitalised adult (≥18 years) patients with laboratory-confirmed COVID-19 were identified in the medical intensive care unit (ICU) or a specialised non-ICU COVID-19 floor in our hospital. Asymptomatic, non-hospitalised controls were recruited as a comparator group for biomarkers that did not have a reference range. We assessed markers of endothelial cell and platelet activation, including von Willebrand Factor (VWF) antigen, soluble thrombomodulin, soluble P-selectin, and soluble CD40 ligand, as well as coagulation factors, endogenous anticoagulants, and fibrinolytic enzymes. We compared the level of each marker in ICU patients, non-ICU patients, and controls, where applicable. We assessed correlations between these laboratory results with clinical outcomes, including hospital discharge and mortality. Kaplan-Meier analysis was used to further explore the association between biochemical markers and survival.
Findings: 68 patients with COVID-19 were included in the study from April 13 to April 24, 2020, including 48 ICU and 20 non-ICU patients, as well as 13 non-hospitalised, asymptomatic controls. Markers of endothelial cell and platelet activation were significantly elevated in ICU patients compared with non-ICU patients, including VWF antigen (mean 565% [SD 199] in ICU patients vs 278% [133] in non-ICU patients; p<0·0001) and soluble P-selectin (15·9 ng/mL [4·8] vs 11·2 ng/mL [3·1]; p=0·0014). VWF antigen concentrations were also elevated above the normal range in 16 (80%) of 20 non-ICU patients. We found mortality to be significantly correlated with VWF antigen (r = 0·38; p=0·0022) and soluble thrombomodulin (r = 0·38; p=0·0078) among all patients. In all patients, soluble thrombomodulin concentrations greater than 3·26 ng/mL were associated with lower rates of hospital discharge (22 [88%] of 25 patients with low concentrations vs 13 [52%] of 25 patients with high concentrations; p=0·0050) and lower likelihood of survival on Kaplan-Meier analysis (hazard ratio 5·9, 95% CI 1·9-18·4; p=0·0087).
Interpretation: Our findings show that endotheliopathy is present in COVID-19 and is likely to be associated with critical illness and death. Early identification of endotheliopathy and strategies to mitigate its progression might improve outcomes in COVID-19.
Funding: This work was supported by a gift donation from Jack Levin to the Benign Hematology programme at Yale, and the National Institutes of Health.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
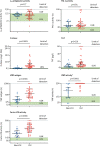
Figure 1
Comparisons of select haemostatic factors in ICU vs non-ICU patients Datapoints indicate individual measurements, whereas horizontal bars show mean (SD) for α2-antiplasmin, VWF antigen, and factor VIII and median (IQR) for PAI-1, D-dimer, TAT, and VWF activity. Green shaded areas indicate the normal range of values. ICU=intensive care unit. ULN=upper limit of normal. LLN=lower limit of normal. FEU=fibrinogen equivalent units. PAI-1=plasminogen activator inhibitor-1. TAT=thrombin-antithrombin complexes. VWF=von Willebrand factor. *Thick horizontal bar denotes patients who had measurements above the limit of detection of the assay.
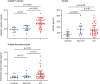
Figure 2
Comparisons of endothelial cell and platelet activation markers in ICU patients, non-ICU patients, and controls Datapoints indicate individual measurements, whereas horizontal bars show mean (SD) for soluble P-selectin and median (IQR) for sCD40L and soluble thrombomodulin. ICU=intensive care unit. sCD40L=soluble CD40 ligand.
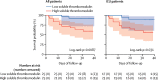
Figure 3
Kaplan–Meier curve of survival and soluble thrombomodulin concentration Data are shown for patients with low soluble thrombomodulin (<3·26 ng/mL) and high soluble thrombomodulin (>3·26 ng/mL). Shaded areas represent 95% CIs. ICU=intensive care unit.
Comment in
- Endothelial cells orchestrate COVID-19 coagulopathy.
O'Sullivan JM, Gonagle DM, Ward SE, Preston RJS, O'Donnell JS. O'Sullivan JM, et al. Lancet Haematol. 2020 Aug;7(8):e553-e555. doi: 10.1016/S2352-3026(20)30215-5. Epub 2020 Jun 30. Lancet Haematol. 2020. PMID: 32619412 Free PMC article. No abstract available.
Similar articles
- ICU Admission Levels of Endothelial Biomarkers as Predictors of Mortality in Critically Ill COVID-19 Patients.
Vassiliou AG, Keskinidou C, Jahaj E, Gallos P, Dimopoulou I, Kotanidou A, Orfanos SE. Vassiliou AG, et al. Cells. 2021 Jan 19;10(1):186. doi: 10.3390/cells10010186. Cells. 2021. PMID: 33477776 Free PMC article. - The coagulopathy, endotheliopathy, and vasculitis of COVID-19.
Iba T, Connors JM, Levy JH. Iba T, et al. Inflamm Res. 2020 Dec;69(12):1181-1189. doi: 10.1007/s00011-020-01401-6. Epub 2020 Sep 12. Inflamm Res. 2020. PMID: 32918567 Free PMC article. Review. - Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19.
Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR, Righy C, Franco S, Souza TML, Kurtz P, Bozza FA, Bozza PT. Hottz ED, et al. Blood. 2020 Sep 10;136(11):1330-1341. doi: 10.1182/blood.2020007252. Blood. 2020. PMID: 32678428 Free PMC article. - Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients.
Zhang Y, Cao W, Jiang W, Xiao M, Li Y, Tang N, Liu Z, Yan X, Zhao Y, Li T, Zhu T. Zhang Y, et al. J Thromb Thrombolysis. 2020 Oct;50(3):580-586. doi: 10.1007/s11239-020-02182-9. J Thromb Thrombolysis. 2020. PMID: 32648093 Free PMC article. - Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy.
Zhang J, Tecson KM, McCullough PA. Zhang J, et al. Rev Cardiovasc Med. 2020 Sep 30;21(3):315-319. doi: 10.31083/j.rcm.2020.03.126. Rev Cardiovasc Med. 2020. PMID: 33070537 Review.
Cited by
- Dysregulation of immunity in COVID-19 and SLE.
Hejazian SS, Hejazian SM, Farnood F, Abedi Azar S. Hejazian SS, et al. Inflammopharmacology. 2022 Oct;30(5):1517-1531. doi: 10.1007/s10787-022-01047-2. Epub 2022 Aug 26. Inflammopharmacology. 2022. PMID: 36028612 Free PMC article. Review. - Dying of VOC-202012/01 - multimodal investigations in a death case of the SARS-CoV-2 variant.
Heinrich F, Romich C, Zimmermann T, Kniep I, Fitzek A, Steurer S, Glatzel M, Nörz D, Günther T, Czech-Sioli M, Fischer N, Grundhoff A, Lütgehetmann M, Ondruschka B. Heinrich F, et al. Int J Legal Med. 2022 Jan;136(1):193-202. doi: 10.1007/s00414-021-02618-8. Epub 2021 Jun 5. Int J Legal Med. 2022. PMID: 34089348 Free PMC article. - Is the Endothelium the Missing Link in the Pathophysiology and Treatment of COVID-19 Complications?
Castro P, Palomo M, Moreno-Castaño AB, Fernández S, Torramadé-Moix S, Pascual G, Martinez-Sanchez J, Richardson E, Téllez A, Nicolas JM, Carreras E, Richardson PG, Badimon JJ, Escolar G, Diaz-Ricart M. Castro P, et al. Cardiovasc Drugs Ther. 2022 Jun;36(3):547-560. doi: 10.1007/s10557-021-07207-w. Epub 2021 Jun 7. Cardiovasc Drugs Ther. 2022. PMID: 34097193 Free PMC article. Review. - Covid-19: The Rollercoaster of Fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes.
Grobler C, Maphumulo SC, Grobbelaar LM, Bredenkamp JC, Laubscher GJ, Lourens PJ, Steenkamp J, Kell DB, Pretorius E. Grobler C, et al. Int J Mol Sci. 2020 Jul 21;21(14):5168. doi: 10.3390/ijms21145168. Int J Mol Sci. 2020. PMID: 32708334 Free PMC article. Review. - Hemostatic alterations in COVID-19.
Peyvandi F, Artoni A, Novembrino C, Aliberti S, Panigada M, Boscarino M, Gualtierotti R, Rossi F, Palla R, Martinelli I, Grasselli G, Blasi F, Tripodi A. Peyvandi F, et al. Haematologica. 2021 May 1;106(5):1472-1475. doi: 10.3324/haematol.2020.262634. Haematologica. 2021. PMID: 32855280 Free PMC article. No abstract available.
References
- Johns Hopkins University Coronavirus resource center. COVID-19 case tracker. https://coronavirus.jhu.edu
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL115247/HL/NHLBI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R01 HL142818/HL/NHLBI NIH HHS/United States
- R56 HL136715/HL/NHLBI NIH HHS/United States
- U54 DK106857/DK/NIDDK NIH HHS/United States
- T32 GM007205/GM/NIGMS NIH HHS/United States
- R01 HL125897/HL/NHLBI NIH HHS/United States
- R01 HL150515/HL/NHLBI NIH HHS/United States
- R01 HL122815/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous