Lower cardiorenal risk with sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes without cardiovascular and renal diseases: A large multinational observational study - PubMed (original) (raw)
Observational Study
doi: 10.1111/dom.14189. Epub 2020 Sep 28.
Johan Bodegard 2, Amitava Banerjee 3 4, Dae Jung Kim 5, Anna Norhammar 6 7, Jan W Eriksson 8, Marcus Thuresson 9, Suguru Okami 10, Kyoung Hwa Ha 5, Nils Kossack 11, Jil Billy Mamza 12, Ruiqi Zhang 12, Toshitaka Yajima 10, Issei Komuro 13, Takashi Kadowaki 14
Affiliations
- PMID: 32893440
- PMCID: PMC7756303
- DOI: 10.1111/dom.14189
Observational Study
Lower cardiorenal risk with sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes without cardiovascular and renal diseases: A large multinational observational study
Kåre I Birkeland et al. Diabetes Obes Metab. 2021 Jan.
Abstract
Aims: We compared the new use of sodium-glucose cotransporter-2 inhibitor (SGLT2i) versus dipeptidyl peptidase-4 inhibitor (DPP4i) and the risk of cardiorenal disease, heart failure (HF) or chronic kidney disease (CKD), in patients with type 2 diabetes without a history of prevalent cardiovascular and renal disease, defined as cardiovascular and renal disease (CVRD) free, managed in routine clinical practice.
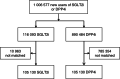
Materials and methods: In this observational cohort study, patients were identified from electronic health records from England, Germany, Japan, Norway, South Korea and Sweden, during 2012-2018. In total, 1 006 577 CVRD-free new users of SGLT2i or DPP4i were propensity score matched 1:1. Unadjusted Cox regression was used to estimate hazard ratios (HRs) for outcomes: cardiorenal disease, HF, CKD, stroke, myocardial infarction (MI), cardiovascular and all-cause mortality.
Results: Baseline characteristics were well balanced between the treatment groups (n = 105 130 in each group) with total follow-up of 187 955 patient years. Patients had a mean age of 56 years, 43% were women and they were indexed between 2013 and 2018. The most commonly used agents were dapagliflozin (91.7% of exposure time) and sitagliptin/linagliptin (55.0%), in the SGLT2i and DPP4i, groups, respectively. SGLT2i was associated with lower risk of cardiorenal disease, HF, CKD, all-cause and cardiovascular mortality; HR (95% confidence interval), 0.56 (0.42-0.74), 0.71 (0.59-0.86), 0.44 (0.28-0.69), 0.67 (0.59-0.77), and 0.61 (0.44-0.85), respectively. No differences were observed for stroke [0.87 (0.69-1.09)] and MI [0.94 (0.80-1.11)].
Conclusion: In this multinational observational study, SGLT2i was associated with a lower risk of HF and CKD versus DPP4i in patients with type 2 diabetes otherwise free from both cardiovascular and renal disease.
Keywords: DPP-IV inhibitor; SGLT2 inhibitor; dapagliflozin; diabetic nephropathy; heart failure; observational study.
© 2020 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
KIB has received grants to his institution from AstraZeneca for this study and for lectures and consulting from Novo Nordisk, Sanofi, Lilly, Boehringer Ingelheim and Merck Sharp & Dohme. JB holds a full‐time position at AstraZeneca as an epidemiologist. AB acknowledges research support from NIHR, BMA, UKRI, HDR UK, EU and AstraZeneca. DJK has received research grant support from LG Life Sciences, Handok, Boehringer Ingelheim, and AstraZeneca; and has received speaker fees from Novo Nordisk, Novartis Korea, Boehringer Ingelheim, Lilly Korea, Handok, MSD, Hanmi and AstraZeneca. AN has received honoraria from MSD, Astra Zeneca, Eli Lilly, Boehringer Ingelheim and Novo Nordisk. JWE has received honoraria or research grants from AstraZeneca, NovoNordisk, Bayer, Sanofi and MSD. MT is employed by an independent statistical consultant company, Statisticon AB, Uppsala, Sweden, of which AstraZeneca Nordic‐Baltic is a client. SO is a full‐time employee of AstraZeneca. KHH has received research grant support from LG Life Sciences, Handok, Boehringer Ingelheim and AstraZeneca. NK is an employee of Wissenschaftliches Institut für Gesundheitsökonomie und Gesundheitssystemforschung and conducted work on behalf of Kantar Health. JBM, RZ and TY are full‐time employees of AstraZeneca. IK received grants from Astellas Pharma Inc., Boehringer Ingelheim Japan, Kowa Pharmaceutical Co. Ltd, Daiichi Sankyo Company Limited., Mitsubishi Tanabe Pharma Corporation, Shionogi & Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Pfizer Japan Inc., Takeda Pharmaceutical Company Limited., Toa Eiyo Ltd, Honorarium: Astellas Pharma Inc., Boehringer Ingelheim Japan, Kowa Pharmaceutical Co. Ltd, Daiichi Sankyo Company Limited., Mitsubishi Tanabe Pharma Corporation, Shionogi & Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Pfizer Japan Inc., Takeda Pharmaceutical Company Limited., Toa Eiyo Ltd. TK received grants from Asahi Mutual Life Insurance Co., Boehringer Ingelheim Japan, Daiichi Sankyo Company Limited, Kowa Pharmaceutical Co. Ltd, Mitsubishi Tanabe Pharma Corporation, MSD K.K., Novo Nordisk Pharma Ltd, Sanofi K.K., Takeda Pharmaceutical Company Limited. Lecture/other fees: AstraZeneca K.K., Astellas Pharma Inc., Boehringer Ingelheim Japan, Daiichi Sankyo Company Limited., Eli Lilly Japan K.K., Kowa Pharmaceutical Co. Ltd, Kyowa Hakko Kirin Co., Ltd, Mitsubishi Tanabe Pharma Corporation, MSD K.K., Ono Pharmaceutical Company, Ltd, Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd, Sanwa Kagaku Kenkyusho Co. Ltd, Taisho Pharmaceutical Co., Ltd and Takeda Pharmaceutical Company Limited.
Figures
FIGURE 1
Patient flow‐chart of patients with type 2 diabetes without cardiovascular or renal disease. DPP4i, dipeptidyl peptidase‐4 inhibitor; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor
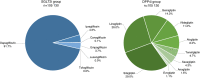
FIGURE 2
Distribution of follow‐up time for the different SGLT2i and DPP4i types. DPP4i, dipeptidyl peptidase‐4 inhibitor; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor
FIGURE 3
Risks of cardiorenal disease, cardiovascular disease and death in patients with type 2 diabetes free from cardiovascular and renal disease. ER, event rates; PY, patient‐years; CKD, chronic kidney disease; DPP4i, dipeptidyl peptidase‐4 inhibitor; HF, heart failure; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor
FIGURE 4
Subgroup analyses of cardiorenal disease in patients with type 2 diabetes free from cardiovascular and renal disease. ER, event rates; PY, patient‐years; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; DPP4i, dipeptidyl peptidase‐4 inhibitor; loop diuretics, high ceiling diuretics; RAASi, renin angiotensin aldosterone system inhibitor; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor
Similar articles
- The risk of new-onset atrial fibrillation in patients with type 2 diabetes mellitus treated with sodium glucose cotransporter 2 inhibitors versus dipeptidyl peptidase-4 inhibitors.
Ling AW, Chan CC, Chen SW, Kao YW, Huang CY, Chan YH, Chu PH. Ling AW, et al. Cardiovasc Diabetol. 2020 Nov 6;19(1):188. doi: 10.1186/s12933-020-01162-w. Cardiovasc Diabetol. 2020. PMID: 33158436 Free PMC article. - Roles for SGLT2 Inhibitors in Cardiorenal Disease.
Green JB, McCullough PA. Green JB, et al. Cardiorenal Med. 2022;12(3):81-93. doi: 10.1159/000524906. Epub 2022 Jul 14. Cardiorenal Med. 2022. PMID: 35835083 Review. - Combination of sodium-glucose cotransporter 2 inhibitor and dipeptidyl peptidase-4 inhibitor in type 2 diabetes: a systematic review with meta-analysis.
Min SH, Yoon JH, Moon SJ, Hahn S, Cho YM. Min SH, et al. Sci Rep. 2018 Mar 13;8(1):4466. doi: 10.1038/s41598-018-22658-2. Sci Rep. 2018. PMID: 29535389 Free PMC article.
Cited by
- Cardiovascular Benefits With Favorable Renal, Amputation and Hypoglycemic Outcomes of SGLT-2 Inhibitors in Type 2 Diabetes From the Asian Perspective: A Population-Based Cohort Study and Systematic Review.
Yang CT, Peng ZY, Chen YC, Ou HT, Kuo S. Yang CT, et al. Front Endocrinol (Lausanne). 2022 Mar 7;13:836365. doi: 10.3389/fendo.2022.836365. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35330915 Free PMC article. - Dapagliflozin treatment of patients with chronic kidney disease without diabetes across different albuminuria levels (OPTIMISE-CKD).
Svensson MK, Tangri N, Bodegård J, Adamsson Eryd S, Thuresson M, Sofue T. Svensson MK, et al. Clin Kidney J. 2024 Apr 4;17(8):sfae100. doi: 10.1093/ckj/sfae100. eCollection 2024 Aug. Clin Kidney J. 2024. PMID: 39165293 Free PMC article. - Differences in outcomes of hospitalizations for heart failure after SGLT2 inhibitor treatment: effect modification by atherosclerotic cardiovascular disease.
Shao SC, Chang KC, Lin SJ, Chang SH, Hung MJ, Chan YY, Lai EC. Shao SC, et al. Cardiovasc Diabetol. 2021 Oct 23;20(1):213. doi: 10.1186/s12933-021-01406-3. Cardiovasc Diabetol. 2021. PMID: 34688282 Free PMC article. - Elucidating the risk of cardiopulmonary consequences of an exacerbation of COPD: results of the EXACOS-CV study in Germany.
Vogelmeier CF, Rhodes K, Garbe E, Abram M, Halbach M, Müllerová H, Kossack N, Timpel P, Kolb N, Nordon C. Vogelmeier CF, et al. BMJ Open Respir Res. 2024 Mar 30;11(1):e002153. doi: 10.1136/bmjresp-2023-002153. BMJ Open Respir Res. 2024. PMID: 38555102 Free PMC article.
References
- Thrainsdottir IS, Aspelund T, Thorgeirsson G, et al. The association between glucose abnormalities and heart failure in the population‐based Reykjavik study. Diabetes Care. 2005;28(3):612‐616. - PubMed
- Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis. 2019;62(4):298‐302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous