Withaferin A activates TRIM16 for its anti-cancer activity in melanoma - PubMed (original) (raw)
Withaferin A activates TRIM16 for its anti-cancer activity in melanoma
Zsuzsanna Nagy et al. Sci Rep. 2020.
Abstract
Although selective BRAF inhibitors and novel immunotherapies have improved short-term treatment responses in metastatic melanoma patients, acquired resistance to these therapeutics still represent a major challenge in clinical practice. In this study, we evaluated the efficacy of Withaferin A (WFA), derived from the medicinal plant Withania Somnifera, as a novel therapeutic agent for the treatment of melanoma. WFA showed selective toxicity to melanoma cells compared to non-malignant cells. WFA induced apoptosis, significantly reduced cell proliferation and inhibited migration of melanoma cells. We identified that repression of the tumour suppressor TRIM16 diminished WFA cytotoxicity, suggesting that TRIM16 was in part responsible for the cytotoxic effects of WFA in melanoma cells. Together our data indicates that WFA has potent cytopathic effects on melanoma cells through TRIM16, suggesting a potential therapeutic application of WFA in the disease.
Conflict of interest statement
The authors declare no competing interests.
Figures
Figure 1
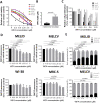
WFA has selective toxicity to melanoma cells compared with fibroblast cell lines. (A), A panel of melanoma (MelJD, MelCV, G361, A375 and MM200) cells and normal fibroblasts (WI-38, MRC-5) were treated with increasing concentrations (0–5 μM) of Withaferin A (WFA) for 48 h and cell viability was measured using the Alamar Blue assay. (B), Average IC50 for WFA-treated melanoma cell lines and normal fibroblasts. Melanoma cell lines (MelJD and MelCV) and normal fibroblasts (WI-38, and MRC-5) were treated with increasing concentrations (0–5 μM) of WFA for 48 h, followed by (C), BrdU cell proliferation assay (D), MITOPROBE DILC1(5) measurements. (E), Flow cytometry results of MelJD and MelCV cells treated with increasing concentrations of WFA, then stained with Annexin-V-FITC/7AAD. Results are mean ± SEM, data was normalized to vehicle control treatment group.
Figure 2
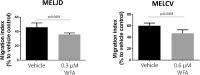
WFA treatment decreases melanoma cell migration. MelJD and MelCV cells treated with indicated concentrations of WFA for 18 h. Results are mean ± SEM, data was normalized to vehicle control treatment group.
Figure 3
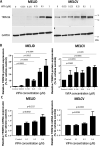
WFA treatment induces TRIM16 protein expression. (A), Western blot images of TRIM16 and GAPDH protein expression in MelJD and MelCV cells treated with WFA at increasing concentrations. The full-length blots are presented in the Supplementary Fig. 3A. (B), The level of TRIM16 protein expression measured by densitometry quantification. (C), RT-qPCR analysis of TRIM16 mRNA expression in MelJD and MelCV cells treated with WFA at increasing concentrations. Results are mean ± SEM, data was normalized to vehicle control treatment group.
Figure 4
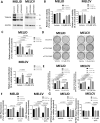
Knock-down of TRIM16 protects melanoma cells against WFA. (A), Western blot analysis of TRIM16 expression in MelJD and MelCV cells following TRIM16 knock-down. GAPDH was used as internal control. The full-length blots are presented in the Supplementary Fig. 3B. MelJD and MelCV cells were transfected with control siRNA (siControl) or TRIM16 siRNAs (siTRIM16) for 24 h, then treated with WFA for additional 48 h followed by (B), Alamar Blue cell viability and (C), and BrdU cell proliferation measurements. Results are mean ± SEM, differences in cell viability and proliferation were compared to the vehicle (DMSO) treated siRNA control cells. (D), MelJD and MelCV cells expressing TRIM16 siRNAs (siTRIM16) or control siRNA (siControl) were seeded for colony formation assay and treated with indictaed concentrations of WFA for 72 h then allowed for colonies to form for 10 days, followed by crystal violet staining. (E), Quantification of colony formation assay based on crystal violet absorbance (590 nm). Differences in colony formation were compared to the vehicle treated control siRNA. MelJD and MelCV cells were transfected with control siRNA (siControl) or TRIM16 siRNAs (siTRIM16) for 24 h, then treated with WFA for additional 48 h followed by (F,) MITOPROBE DILC1(5) and (G), SYTOX Green measurements. Results are mean ± SEM, differences in cell viability and proliferation were compared to the vehicle control siRNA control cells.
Similar articles
- Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2.
Mayola E, Gallerne C, Esposti DD, Martel C, Pervaiz S, Larue L, Debuire B, Lemoine A, Brenner C, Lemaire C. Mayola E, et al. Apoptosis. 2011 Oct;16(10):1014-27. doi: 10.1007/s10495-011-0625-x. Apoptosis. 2011. PMID: 21710254 - TRIM16 inhibits proliferation and migration through regulation of interferon beta 1 in melanoma cells.
Sutton SK, Koach J, Tan O, Liu B, Carter DR, Wilmott JS, Yosufi B, Haydu LE, Mann GJ, Thompson JF, Long GV, Liu T, McArthur G, Zhang XD, Scolyer RA, Cheung BB, Marshall GM. Sutton SK, et al. Oncotarget. 2014 Oct 30;5(20):10127-39. doi: 10.18632/oncotarget.2466. Oncotarget. 2014. PMID: 25333256 Free PMC article. - Withaferin A suppresses breast cancer cell proliferation by inhibition of the two-pore domain potassium (K2P9) channel TASK-3.
Zúñiga R, Concha G, Cayo A, Cikutović-Molina R, Arevalo B, González W, Catalán MA, Zúñiga L. Zúñiga R, et al. Biomed Pharmacother. 2020 Sep;129:110383. doi: 10.1016/j.biopha.2020.110383. Epub 2020 Jun 17. Biomed Pharmacother. 2020. PMID: 32563149 - Withaferin A-A Promising Phytochemical Compound with Multiple Results in Dermatological Diseases.
Bungau S, Vesa CM, Abid A, Behl T, Tit DM, Purza AL, Pasca B, Todan LM, Endres L. Bungau S, et al. Molecules. 2021 Apr 21;26(9):2407. doi: 10.3390/molecules26092407. Molecules. 2021. PMID: 33919088 Free PMC article. Review. - Withania Somnifera (Ashwagandha) and Withaferin A: Potential in Integrative Oncology.
Dutta R, Khalil R, Green R, Mohapatra SS, Mohapatra S. Dutta R, et al. Int J Mol Sci. 2019 Oct 25;20(21):5310. doi: 10.3390/ijms20215310. Int J Mol Sci. 2019. PMID: 31731424 Free PMC article. Review.
Cited by
- Withanolide derivatives: natural compounds with anticancer potential offer low toxicity to fertility and ovarian follicles in mice.
Palomino GJQ, Celiz HY, Gomes FDR, Tetaping GM, Novaes MAS, Rocha KAD, Raposo RDS, Rocha RMP, Duarte ABG, Pessoa ODL, Figueiredo JR, de Sá NAR, Rodrigues APR. Palomino GJQ, et al. Anim Reprod. 2024 Oct 21;21(4):e20240027. doi: 10.1590/1984-3143-AR2024-0027. eCollection 2024. Anim Reprod. 2024. PMID: 39494127 Free PMC article. - TRIM3 and TRIM16 as potential tumor suppressors in breast cancer patients.
Roshanazadeh MR, Adelipour M, Sanaei A, Chenane H, Rashidi M. Roshanazadeh MR, et al. BMC Res Notes. 2022 Sep 30;15(1):312. doi: 10.1186/s13104-022-06193-y. BMC Res Notes. 2022. PMID: 36180926 Free PMC article. - Differential response of MDA‑MB‑231 breast cancer and MCF10A normal breast cells to cytoskeletal disruption.
Kwon S, Han SJ, Kim KS. Kwon S, et al. Oncol Rep. 2023 Nov;50(5):200. doi: 10.3892/or.2023.8637. Epub 2023 Sep 29. Oncol Rep. 2023. PMID: 37772386 Free PMC article. - Cancer Metabolism as a Therapeutic Target and Review of Interventions.
Halma MTJ, Tuszynski JA, Marik PE. Halma MTJ, et al. Nutrients. 2023 Oct 1;15(19):4245. doi: 10.3390/nu15194245. Nutrients. 2023. PMID: 37836529 Free PMC article. Review. - A Low Dose Combination of Withaferin A and Caffeic Acid Phenethyl Ester Possesses Anti-Metastatic Potential In Vitro: Molecular Targets and Mechanisms.
Sari AN, Dhanjal JK, Elwakeel A, Kumar V, Meidinna HN, Zhang H, Ishida Y, Terao K, Sundar D, Kaul SC, Wadhwa R. Sari AN, et al. Cancers (Basel). 2022 Feb 3;14(3):787. doi: 10.3390/cancers14030787. Cancers (Basel). 2022. PMID: 35159054 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials