Synaptic Vesicles Dynamics in Neocortical Epilepsy - PubMed (original) (raw)
Synaptic Vesicles Dynamics in Neocortical Epilepsy
Eleonora Vannini et al. Front Cell Neurosci. 2020.
Abstract
Neuronal hyperexcitability often results from an unbalance between excitatory and inhibitory neurotransmission, but the synaptic alterations leading to enhanced seizure propensity are only partly understood. Taking advantage of a mouse model of neocortical epilepsy, we used a combination of photoconversion and electron microscopy to assess changes in synaptic vesicles pools in vivo. Our analyses reveal that epileptic networks show an early onset lengthening of active zones at inhibitory synapses, together with a delayed spatial reorganization of recycled vesicles at excitatory synapses. Proteomics of synaptic content indicate that specific proteins were increased in epileptic mice. Altogether, our data reveal a complex landscape of nanoscale changes affecting the epileptic synaptic release machinery. In particular, our findings show that an altered positioning of release-competent vesicles represent a novel signature of epileptic networks.
Keywords: epilepsy; hyperexcitability; synaptic vesicles; tetanus neurotoxin; visual cortex; visual processing.
Copyright © 2020 Vannini, Restani, Dilillo, McDonnell, Caleo and Marra.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
FIGURE 1
Ultrastructural and functional changes at presynaptic terminals of TeNT-injected mice. (A) Diagrammatic representation of labeling protocol. Visual cortices from mice in Control, Acute, and Chronic groups were infused with FM1-43FX during visual stimulation. Brains were rapidly fixed and sliced to allow photoconversion of FM1-43FX signal before processing for electron microscopy. Individual presynaptic terminals were classified as excitatory (asymmetrical synapses, red) or inhibitory (symmetrical synapses, blue); size, position and numbers of active zone (AZ, yellow), non-released vesicles (open circles) and released vesicles (black circles) were analyzed (scale bars 100 nm). (B) Left: Active zone (AZ) length in Control (gray), Acute (orange), and Chronic (blue) epileptic mice at excitatory synapses; no differences between the groups (Kruskal-Wallis test, p = 0.10). Distribution, median and quartiles shown for each group; Control n = 41; Acute n = 46; Chronic n = 118. Right: Active zone (AZ) length in Control (gray), Acute (orange), and Chronic (blue) epileptic mice at inhibitory synapses (Kruskal-Wallis test, p < 0.01, Control vs. Acute _p_ < 0.01, Control vs. Chronic _p_ > 0.05, Chronic vs. Acute p < 0.01). Distribution, median and quartiles shown for each group; Control _n_ = 15; Acute _n_ = 14; Chronic _n_ = 29. **(C)** Left: Released fraction of synaptic vesicles (labeled vesicles/total vesicles) in Control (gray), Acute (orange), and Chronic (blue) epileptic mice at excitatory synapses; no differences between the groups (Kruskal-Wallis test, _p_ = 0.67). Distribution, median, and quartiles shown for each group; Control _n_ = 47; Acute _n_ = 57; Chronic _n_ = 118. Right: Released fraction of synaptic vesicles of Control (gray), Acute (orange), and Chronic (blue) epileptic mice at inhibitory synapses (Kruskal-Wallis test, _p_ < 0.05, Control vs. Acute _p_ < 0.05, Control vs. Chronic _p_ < 0.01, Chronic vs. Acute _p_ > 0.05). Distribution, median, and quartiles shown for each group; Control n = 16; Acute n = 16; Chronic n = 30.
FIGURE 2
Changes in released vesicles’ docking and spatial organization in chronic phase of epilepsy. (A) Ratio of released vesicles in the docked and undocked population. Left: Diagram and legend for each pie chart. Top: Excitatory synapses’ ratio of released vesicles (darker) in docked (inner pie chart) and undocked population (outer pie chart) in Control (gray), Acute (orange), and Chronic (blue) groups. Only the Chronic group shows a significant difference from expected frequencies based on control observation (Chi-squared test: p < 0.001). Bottom: Inhibitory synapses’ ratio of released vesicles (darker) in docked (inner pie chart) and undocked population (outer pie chart) in control (gray), acute (orange), and chronic (blue) groups. (B) Distance of released or non-released vesicles to the closest point on the active zone. Left: Diagram representing of how distance measures were taken at each synapse. Top: Sigmoid fit and 95% confidence interval of cumulative fraction of distance between released and not-released synaptic vesicles to the active zone at excitatory synapses in Control (gray), Acute (orange), and Chronic (blue) epileptic mice. Bottom: Sigmoid fit and 95% confidence interval of cumulative fraction of distance between released and not-released synaptic vesicles to the active zone at inhibitory synapses in Control (gray), Acute (orange), and Chronic (blue) epileptic mice. Paired _t_-test, Excitatory synapses: Control mice p = 0.0002 (n = 40), Acute mice p = 0.0006 (n = 41), Chronic mice p = 0.298 (n = 112). Paired _t_-test, Inhibitory synapses: Control mice p = 0.06 (n = 14), Acute mice p = 0.135 (n = 13), Chronic mice p = 0.001 (n = 28). (C) 2D histograms of released vesicles distribution at excitatory (top) and inhibitory (bottom) synapses across the three conditions with active zone at the origin of the XY plane. Control (gray), Acute (orange), and Chronic (blue). Each synapse was spatially normalized (_X_- and _Y_-axis) and frequency is plotted on the _Z_-axis. Scale bars: 0.1 normalized size X and Y; 0.1 fraction _Z_-axis.
FIGURE 3
Proteomics analysis of synaptosomes reveal an increase of proteins involved in vesicular positioning. (A,B) Differentially expressed proteins in Control vs. Acute (A) and Chronic epileptic phase (B). Volcano plots are built plotting average ratio of TeNT vs. corresponding control against their _t_-test log _P_-values; significance thresholds: FDR > 0.05 and fold change > 0.6. Proteins significantly upregulated in Acute and Chronic tetanic animals are highlighted, respectively, in orange and light blue; proteins significantly downregulated are in dark gray. Proteins abbreviations are Dkk3, Dickkopf-related protein 3; Sema4a, Semaphorin 4A; Cpe, carboxypeptidase e; Chgb, chromogranin b; Syt5, synaptotagmin5; VAMP1, Vesicle-associated membrane protein 1; VAMP2, Vesicle-associated membrane protein 2; C1qc, Complement C1q C Chain. (C) Proportion of presynaptic terminals containing Dense Core Vesicles in different non-overlapping sampled areas of Control (gray; n = 20), Acute (orange; n = 29), and Chronic (blue; n = 15) groups. No differences between groups (One Way ANOVA, p = 0.2869). Data are represented as mean ± SEM. Inset, a representative image of Dense Core Vesicles. (D) Right: Distribution of distances of non-released vesicles from active zone at excitatory synapses in Chronic (gray; n = 2140), Acute (orange; n = 2503), and Chronic (blue; n = 5705) groups (One-way ANOVA; F = 238.15, p < 0.0001, Control vs. Actute: _p_ < 0.0001; Control vs. Chronic: _p_ < 0.0001). Left: Distribution of distances of non-released vesicles from active zone at inhibitory synapses in Chronic (gray; _n_ = 543), Acute (orange; _n_ = 717), and Chronic (blue; _n_ = 1520) groups (_F_ = 75.57, _p_ < 0.0001, Control vs. Actute: _p_ < 0.0001; Control vs. Chronic: _p_ > 0.05).
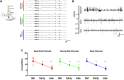
FIGURE 4
Acute inhibition of Carboxypeptidase (CPE) decreases hyperexcitability in TeNT-injected mice. (A) Left: diagram of experimental design: a 16-channel silicone probe was used to record LFP in different layers of the primary visual cortex, channels were analyzed in three groups according to their recording sites in relation to the surface of the cortex: the five most superficial, the five deepest, and the six intermediate channels. GEMSA was applied locally to inhibit CPE activity. Right: Examples of LFP traces obtained with a 16-channels probe from the visual cortex of an Acute epileptic mouse. (B) LFP traces of an Acute epileptic mouse before (baseline, top) and after GEMSA administration at two different time points: early (5–10 min) and late (10–20 min). (C) Coastline analysis of LFP signals recorded before (baseline) and after GEMSA administration at early and late time points. The analysis was differentially performed for superficial (left, red), intermediate (middle, green), and deep (right, blue) channels (Two-way ANOVA, Channel factor p > 0.05, Time factor p < 0.001; Baseline vs. Early: p < 0.01, Baseline vs. Late: p < 0.001, Early vs. Late: p < 0.001, n = 4). The mean, SEM, and value of individual recordings are shown for each group. ***p < 0.001.
Similar articles
- Synaptic alterations and neuronal firing in human epileptic neocortical excitatory networks.
Bod R, Tóth K, Essam N, Tóth EZ, Erõss L, Entz L, Bagó AG, Fabó D, Ulbert I, Wittner L. Bod R, et al. Front Synaptic Neurosci. 2023 Aug 10;15:1233569. doi: 10.3389/fnsyn.2023.1233569. eCollection 2023. Front Synaptic Neurosci. 2023. PMID: 37635750 Free PMC article. - Disruption of layer-specific visual processing in a model of focal neocortical epilepsy.
Panarese A, Vissani M, Meneghetti N, Vannini E, Cracchiolo M, Micera S, Caleo M, Mazzoni A, Restani L. Panarese A, et al. Cereb Cortex. 2023 Mar 21;33(7):4173-4187. doi: 10.1093/cercor/bhac335. Cereb Cortex. 2023. PMID: 36089833 - Complexin Mutants Reveal Partial Segregation between Recycling Pathways That Drive Evoked and Spontaneous Neurotransmission.
Sabeva N, Cho RW, Vasin A, Gonzalez A, Littleton JT, Bykhovskaia M. Sabeva N, et al. J Neurosci. 2017 Jan 11;37(2):383-396. doi: 10.1523/JNEUROSCI.1854-16.2016. J Neurosci. 2017. PMID: 28077717 Free PMC article. - Cellular abnormalities and synaptic plasticity in seizure disorders of the immature nervous system.
Swann JW, Hablitz JJ. Swann JW, et al. Ment Retard Dev Disabil Res Rev. 2000;6(4):258-67. doi: 10.1002/1098-2779(2000)6:4<258::AID-MRDD5>3.0.CO;2-H. Ment Retard Dev Disabil Res Rev. 2000. PMID: 11107191 Review. - Plasticity of Hippocampal Excitatory-Inhibitory Balance: Missing the Synaptic Control in the Epileptic Brain.
Bonansco C, Fuenzalida M. Bonansco C, et al. Neural Plast. 2016;2016:8607038. doi: 10.1155/2016/8607038. Epub 2016 Feb 24. Neural Plast. 2016. PMID: 27006834 Free PMC article. Review.
Cited by
- Role of Clathrin and Dynamin in Clathrin Mediated Endocytosis/Synaptic Vesicle Recycling and Implications in Neurological Diseases.
Prichard KL, O'Brien NS, Murcia SR, Baker JR, McCluskey A. Prichard KL, et al. Front Cell Neurosci. 2022 Jan 18;15:754110. doi: 10.3389/fncel.2021.754110. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35115907 Free PMC article. Review. - Glutamate's Secret Interictal Life.
Cullen ER, Weston MC. Cullen ER, et al. Epilepsy Curr. 2021 Sep 17;21(6):460-462. doi: 10.1177/15357597211043728. eCollection 2021 Nov-Dec. Epilepsy Curr. 2021. PMID: 34924859 Free PMC article. No abstract available. - The role of peptidyl-prolyl isomerase Pin1 in neuronal signaling in epilepsy.
Chen Y, Hou X, Pang J, Yang F, Li A, Lin S, Lin N, Lee TH, Liu H. Chen Y, et al. Front Mol Neurosci. 2022 Oct 11;15:1006419. doi: 10.3389/fnmol.2022.1006419. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36304997 Free PMC article. Review. - Elevated amyloid beta disrupts the nanoscale organization and function of synaptic vesicle pools in hippocampal neurons.
Biasetti L, Rey S, Fowler M, Ratnayaka A, Fennell K, Smith C, Marshall K, Hall C, Vargas-Caballero M, Serpell L, Staras K. Biasetti L, et al. Cereb Cortex. 2023 Feb 7;33(4):1263-1276. doi: 10.1093/cercor/bhac134. Cereb Cortex. 2023. PMID: 35368053 Free PMC article. - Presynaptic antiseizure medications - basic mechanisms and clues for their rational combinations.
Czapińska-Ciepiela EK, Łuszczki J, Czapiński P, Czuczwar SJ, Lasoń W. Czapińska-Ciepiela EK, et al. Pharmacol Rep. 2024 Aug;76(4):623-643. doi: 10.1007/s43440-024-00603-7. Epub 2024 May 22. Pharmacol Rep. 2024. PMID: 38776036 Free PMC article. Review.
References
- Bernard C. (2010). Alterations in synaptic function in epilepsy. Epilepsia 51:42 10.1111/j.1528-1167.2010.02828.x - DOI
- Bromfield E. B., Cavazos J. E. (2006). Basic Mechanisms Underlying Seizures and Epilepsy - An Introduction to Epilepsy - NCBI Bookshelf. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK2510/ (accessed March 13, 2020)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases