The protective effects of granulocyte-macrophage colony-stimulating factor against radiation-induced lung injury - PubMed (original) (raw)
The protective effects of granulocyte-macrophage colony-stimulating factor against radiation-induced lung injury
Dan Hu et al. Transl Lung Cancer Res. 2020 Dec.
Abstract
Background: Radiation-induced lung injury (RILI) is a common complication of thoracic cancer radiation therapy. Currently, there is no effective treatment for RILI. RILI is associated with chronic inflammation, this injury is perpetuated by the stimulation of chemokines and proinflammatory cytokines. Recent studies have demonstrated that granulocyte-macrophage colony-stimulating factor (GM-CSF) plays a pivotal role in inflammation and fibrosis. This study aimed to investigate the protective effect of GM-CSF against the development of RILI in lung tissue.
Method: First, a single fraction of radiation at a dose of 16 Gy was targeted at the entire thorax of wild-type (WT) C57BL/6 mice and GM-CSF-/- mice to induce RILI. Second, we detected the radioprotective effects of GM-CSF by measuring the inflammatory biomarkers and fibrosis alteration on radiated lung tissues. Furthermore, we investigated the potential mechanism of GM-CSF protective effects in RILI.
Results: The GM-CSF-/- mice sustained more severe RILI than the WT mice. RILI was significantly alleviated by GM-CSF treatment. Intraperitoneally administered GM-CSF significantly inhibited inflammatory cytokine production and decreased epithelial-mesenchymal transition (EMT) in the RILI mouse model.
Conclusions: GM-CSF was shown to be an important modulator of RILI through regulating inflammatory cytokines, which provides a new strategy for the prevention and treatment of RILI.
Keywords: GM-CSF-deficient mice; Radiation-induced lung injury (RILI); TGF-β1; epithelial-mesenchymal transition (EMT); granulocyte-macrophage colony-stimulating factor (GM-CSF).
2020 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-1272). The authors have no conflicts of interest to declare.
Figures
Figure 1
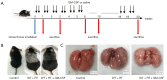
Establishment of a thoracic radiation mouse model. (A) Intervention with GM-CSF in the experimental mouse model of whole thorax radiation. C57BL/6 WT mice were intraperitoneally injected with GM-CSF or vehicle (n=6 per group) treatment twice weekly after radiation. Lung tissues were harvested at the indicated time points for the following analysis. (B) Pattern of color change in the hair of the radiated thoracic region at 20 weeks after radiation. (C) The lung phenotype among the control, vehicle, and GM-CSF intervention groups at 20 weeks post radiation. Scale bar represents 300 mm. GM-CSF, granulocyte-macrophage colony-stimulating factor; WT, wild-type; RT, radiation.
Figure 2
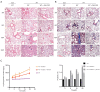
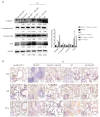
Pulmonary fibrosis caused by radiation was attenuated by intraperitoneal administration of GM-CSF. (A) Lung sections of WT mice from different groups were subjected to H&E staining at 3, 6, 10, and 20 weeks after radiation. Lung bleeding sites and alveolitis were showed with arrows. Representative images are shown (n=6 per group). Scale bar represents 300 μm. (B) Masson trichrome staining of collagen in lung sections from WT mice in the normal non-radiated, radiation only, and GM-CSF treatment groups at 3, 6, 10, and 20 weeks after radiation. Abnormal collagen deposition were showed with arrows. Representative images are shown (n=6 per group). Bar represents 300 μm. The down panel is quantification of Masson’s trichrome staining for collagen in lung sections from different groups. The fibrotic area is presented as percentage numbers. (C) Hyp content was measured between different groups. Error bar indicates mean ± standard deviation (n=6; *, P<0.05; **, P<0.01; ***, P<0.001, WT + radiation vs. WT + radiation + GM-CSF). GM-CSF, granulocyte-macrophage colony-stimulating factor; WT, wild-type; H&E, hematoxylin and eosin; Hyp, hydroxyproline; RT, radiation.
Figure 3
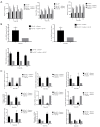
Effect of GM-CSF on inflammatory cytokines in the radiation mouse model. (A) ELISA analysis of the serum levels of IL-6, TNF-α, and IL-1β in mice from different groups in the radiation-induced injury mouse model at 1, 3, 6, 10, and 20 weeks post radiation. (B) qRT-PCR analysis of IL-6, TNF-α, and IL-1β mRNA levels in the lung tissues of mice from different groups at different time points (n=6 per group). (C) qRT-PCR analysis of EMT-related markers. The target gene mRNA levels were normalized to GAPDH (n=6 per group). *, P<0.05; **, P<0.01; ***, P<0.001. GM-CSF, granulocyte-macrophage colony-stimulating factor; ELISA, enzyme-linked immunosorbent assay; qRT-PCR, quantitative real-time polymerase chain reaction; EMT, epithelial-mesenchymal transition; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; WT, wild-type.
Figure 4
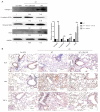
Effect of GM-CSF on mesenchymal markers. (A) Lung tissues were subjected to western blot analysis to detect the expression of collagen-1, ECA, VIM, and Snail at 20 weeks after radiation. GAPDH was used as an internal reference for relative quantification. (B) Relative protein expression of ECA, VIM, and TGF-β was measured by IHC. Scale bars represent 200 μm (20×) and 100 μm (40×). Error bar indicates mean ± standard deviation (n=6; **, P<0.01). GM-CSF, granulocyte-macrophage colony-stimulating factor; ECA, E-cadherin; VIM, vimentin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IHC, immunohistochemistry; WT, wild-type; con, control.
Figure 5
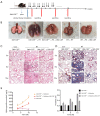
Effect of GM-CSF on radiation-induced pneumonia and collagen deposition in GM-CSF–/– mice. (A) GM-CSF–/– mice were radiated with a single fraction of 16-Gy photons targeted at the entire thorax. GM-CSF–/– mice were intraperitoneally injected with 10 μg/kg GM-CSF (n=6 per group) twice weekly after radiation therapy, while the matched group received the same volume of vehicle instead. (B) GM-CSF reduced radiation-induced hyperemia and edema in pulmonary tissue at 10 weeks post-radiation. Representative images of GM-CSF–/– mouse lung tissues are shown from the control group, single radiation group, and GM-CSF-treated group. (C,D) GM-CSF mitigated radiation-induced pneumonia and collagen deposition in lung tissues. The arrows showed the damage to the pulmonary alveolar architecture and abnormal collagen deposition. Representative images of H&E and Masson staining of lung tissue sections at 3–10 weeks post radiation (bar represents 300 μm). The down panel of (D) is quantification of collagen deposition in slides from lung tissues. Values are given as mean ± standard deviation (n=6), *, P<0.05 and ***, P<0.001 versus single radiation group. (E) Lung tissues of GM-CSF–/– mice from 3, 6, 10 weeks after radiation was analyzed for Hyp content. Error bar indicates mean ± standard deviation (n=6; *, P<0.05; **, P<0.01; ***, P<0.001, GM-CSF–/– + radiation vs. GM-CSF–/– + radiation + GM-CSF). GM-CSF, granulocyte-macrophage colony-stimulating factor; H&E, hematoxylin and eosin; Hyp, hydroxyproline.
Figure 6
Effect of GM-CSF on lung inflammatory cytokines in radiated GM-CSF–/– mice. (A) GM-CSF reduced the levels of profibrotic cytokines in GM-CSF–/– mice with lung injury after radiation. Cytokine concentration in the serum of radiated mice with different treatments. (B) Relative mRNA levels of inflammatory markers including IL-6, IL-1β, and TNF-α in the lungs were calculated in different groups (n=6 per group). (C) Relative mRNA levels of EMT-related markers including TGF-β1, MMP-9, epithelial cell markers including Occuludin, E-cadherin, and SFTPC, and mesenchymal cell markers including vimentin, were calculated in different groups (n=6 per group). *, P<0.05; **, P<0.01; ***, P<0.001. GM-CSF, granulocyte-macrophage colony-stimulating factor; EMT, epithelial-mesenchymal transition.
Figure 7
Effect of GM-CSF on EMT in radiated pulmonary fibrosis. (A) Western blot analysis of EMT markers in radiated lung tissues with/without GM-CSF treatment at 10 weeks. Quantification analysis of raw density of Western blot figures are measured in different groups. (B) GM-CSF reduced the expression levels of ECA, VIM and TGF-β1. Representative images of IHC staining of ECA, VIM, and TGF-β1 are assessed in lung tissues from different groups at 10 weeks. Error bar indicates mean ± standard deviation (n=6; **, P<0.01). GM-CSF, granulocyte-macrophage colony-stimulating factor; EMT, epithelial-mesenchymal transition; ECA, E-cadherin; VIM, vimentin; IHC, immunohistochemistry.
Similar articles
- Ethyl pyruvate alleviates radiation-induced lung injury in mice.
Chen B, Na F, Yang H, Li R, Li M, Sun X, Hu B, Huang G, Lan J, Xu H, Tong R, Mo X, Xue J, Lu Y. Chen B, et al. Biomed Pharmacother. 2017 Aug;92:468-478. doi: 10.1016/j.biopha.2017.05.111. Epub 2017 May 29. Biomed Pharmacother. 2017. PMID: 28570981 - Protection against acute radiation-induced lung injury: a novel role for the anti-angiogenic agent Endostar.
Zhang K, Yang S, Zhu Y, Mo A, Zhang D, Liu L. Zhang K, et al. Mol Med Rep. 2012 Aug;6(2):309-15. doi: 10.3892/mmr.2012.903. Epub 2012 May 4. Mol Med Rep. 2012. PMID: 22562140 - Targeting granulocyte-macrophage colony-stimulating factor in epithelial and vascular remodeling in experimental eosinophilic esophagitis.
McNamee EN, Biette KA, Hammer J, Harris R, Miyazawa H, Lee JJ, Furuta GT, Masterson JC. McNamee EN, et al. Allergy. 2017 Aug;72(8):1232-1242. doi: 10.1111/all.13105. Epub 2017 Jan 23. Allergy. 2017. PMID: 27926989 - Gene transfer for cytokine functional studies in the lung: the multifunctional role of GM-CSF in pulmonary inflammation.
Xing Z, Braciak T, Ohkawara Y, Sallenave JM, Foley R, Sime PJ, Jordana M, Graham FL, Gauldie J. Xing Z, et al. J Leukoc Biol. 1996 Apr;59(4):481-8. doi: 10.1002/jlb.59.4.481. J Leukoc Biol. 1996. PMID: 8613693 Review. - Cytokine, chemokine alterations and immune cell infiltration in Radiation-induced lung injury: Implications for prevention and management.
Guo H, Yu R, Zhang H, Wang W. Guo H, et al. Int Immunopharmacol. 2024 Jan 5;126:111263. doi: 10.1016/j.intimp.2023.111263. Epub 2023 Nov 24. Int Immunopharmacol. 2024. PMID: 38000232 Review.
Cited by
- Emetine dihydrochloride alleviated radiation-induced lung injury through inhibiting EMT.
Wang X, Li M, Yin J, Fang J, Ying Y, Ye T, Zhang F, Ma S, Qin H, Liu X. Wang X, et al. J Cell Mol Med. 2023 Dec;27(23):3839-3850. doi: 10.1111/jcmm.17959. Epub 2023 Sep 18. J Cell Mol Med. 2023. PMID: 37723905 Free PMC article. - Towards unravelling biological mechanisms behind radiation-induced oral mucositis via mass spectrometry-based proteomics.
Subedi P, Huber K, Sterr C, Dietz A, Strasser L, Kaestle F, Hauck SM, Duchrow L, Aldrian C, Monroy Ordonez EB, Luka B, Thomsen AR, Henke M, Gomolka M, Rößler U, Azimzadeh O, Moertl S, Hornhardt S. Subedi P, et al. Front Oncol. 2023 Jun 13;13:1180642. doi: 10.3389/fonc.2023.1180642. eCollection 2023. Front Oncol. 2023. PMID: 37384298 Free PMC article. - METTL3 Mediates Epithelial-Mesenchymal Transition by Modulating FOXO1 mRNA N6 -Methyladenosine-Dependent YTHDF2 Binding: A Novel Mechanism of Radiation-Induced Lung Injury.
Feng Y, Yuan P, Guo H, Gu L, Yang Z, Wang J, Zhu W, Zhang Q, Cao J, Wang L, Jiao Y. Feng Y, et al. Adv Sci (Weinh). 2023 Jun;10(17):e2204784. doi: 10.1002/advs.202204784. Epub 2023 Apr 18. Adv Sci (Weinh). 2023. PMID: 37072646 Free PMC article. - Identification of S100A9 as a Potential Inflammation-Related Biomarker for Radiation-Induced Lung Injury.
Liu Y, Wu M, Guo J, Tang Y, Jiang H, Yang B, Wu M, Huang J. Liu Y, et al. J Clin Med. 2023 Jan 17;12(3):733. doi: 10.3390/jcm12030733. J Clin Med. 2023. PMID: 36769382 Free PMC article. - Mesenchymal stem cells in radiation-induced lung injury: From mechanisms to therapeutic potential.
Hou G, Li J, Liu W, Wei J, Xin Y, Jiang X. Hou G, et al. Front Cell Dev Biol. 2022 Dec 12;10:1100305. doi: 10.3389/fcell.2022.1100305. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36578783 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources