DNAJC9 integrates heat shock molecular chaperones into the histone chaperone network - PubMed (original) (raw)
. 2021 Jun 17;81(12):2533-2548.e9.
doi: 10.1016/j.molcel.2021.03.041. Epub 2021 Apr 14.
Hongyu Bao 2, Ivo A Hendriks 3, Massimo Carraro 1, Alberto García-Nieto 4, Yanhong Liu 2, Nazaret Reverón-Gómez 1, Christos Spanos 5, Liu Chen 2, Juri Rappsilber 6, Michael L Nielsen 3, Dinshaw J Patel 7, Hongda Huang 8, Anja Groth 9
Affiliations
- PMID: 33857403
- PMCID: PMC8221569
- DOI: 10.1016/j.molcel.2021.03.041
DNAJC9 integrates heat shock molecular chaperones into the histone chaperone network
Colin M Hammond et al. Mol Cell. 2021.
Abstract
From biosynthesis to assembly into nucleosomes, histones are handed through a cascade of histone chaperones, which shield histones from non-specific interactions. Whether mechanisms exist to safeguard the histone fold during histone chaperone handover events or to release trapped intermediates is unclear. Using structure-guided and functional proteomics, we identify and characterize a histone chaperone function of DNAJC9, a heat shock co-chaperone that promotes HSP70-mediated catalysis. We elucidate the structure of DNAJC9, in a histone H3-H4 co-chaperone complex with MCM2, revealing how this dual histone and heat shock co-chaperone binds histone substrates. We show that DNAJC9 recruits HSP70-type enzymes via its J domain to fold histone H3-H4 substrates: upstream in the histone supply chain, during replication- and transcription-coupled nucleosome assembly, and to clean up spurious interactions. With its dual functionality, DNAJC9 integrates ATP-resourced protein folding into the histone supply pathway to resolve aberrant intermediates throughout the dynamic lives of histones.
Keywords: DNAJC9; HSP40; HSP70; MCM2; TONSL; chromatin replication; heat shock co-chaperone; histone chaperone; nucleosome assembly; transcription.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.M.H., D.J.P., H.H., and A.G. are inventors on a filed patent application covering the therapeutic targeting of TONSL for cancer therapy. A.G. is a co-founder and chief scientific officer (CSO) of Ankrin Therapeutics. A.G. is a member of Molecular Cell’s scientific advisory board.
Figures
Graphical abstract
Figure 1
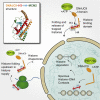
Identification of DNAJC9 as a dual histone chaperone and heat shock co-chaperone (A) Schematic representations of the histone H3-H4 (red and cyan, respectively) binding mode of MCM2 (dark green) and TONSL (yellow) highlighting histone binding mutants (Huang et al., 2015; Saredi et al., 2016) and the experimental strategy for the identification of histone-dependent interactors. Hypothetical histone-dependent interactors (colored light green and orange for MCM2 and TONSL, respectively) and histone-independent interactors (gray) are depicted. (B and C) Mass spectrometry analysis of SILAC labeled pull-downs of wild-type (WT) and histone binding mutant (HBM) forms of MCM2 (B) and TONSL (C) from soluble cell extracts; n = 2 biological replicates. Proteins referred to by human UniProt protein identification code. See also Table S1. (D) Pull-downs of full-length GST-DNAJC9 mixed with pre-assembled H3.3-H4 (top) and MCM2 HBD-H3.3-H4 complexes (bottom). (E) Pull-downs of FLAGHA-tagged histone H3 variants induced by doxycycline (Dox+) compared with control purifications (Dox−) from soluble cell extracts. Western blots representative of n = 2 biological replicates. (F) Plasmid supercoiling assay showing histone chaperone activity of DNAJC9 compared with ASF1A and NASP HBD as positive controls. R, relaxed DNA; S, supercoiled DNA. (G) Schematics depicting the ability of the DNAJC9 N-terminal J domain to stimulate HSP70 catalysis (Han et al., 2007) and the histone-dependent interactions formed between DNAJC9, MCM2, and TONSL.
Figure 2
Structure of DNAJC9 and MCM2 bound simultaneously to an H3.3-H4 dimer (A) Schematic domain architectures of DNAJC9, H3, H4, and MCM2. (B) Pull-downs of GST-DNAJC9 constructs truncated to map the domain of interaction with pre-assembled MCM2 HBD-H3.3-H4 complexes. See Figure S1A for analogous GST-DNAJC9 pull-downs of H3.3-H4 complexes. (C) Structure of the DNAJC9 HBD-H3.3-H4-MCM2 HBD quaternary complex, with DNAJC9 HBD colored in magenta, H3.3 in blue, H4 in green, and MCM2 HBD in pink. See also Table 1 and Figure S1. (D) DNAJC9 HBD (magenta) and MCM2 HBD (pink) wrapping around the H3.3-H4 dimer in surface view colored according to electrostatic potential (red, negatively charged; blue, positively charged). (E) Structural comparison between DNAJC9 HBD-H3.3-H4-MCM2 HBD (colored as in C) and MCM2 HBD-H3.3-H4-ASF1B (silver; PDB:
5BNX
). The αB helix of DNAJC9 HBD forms a steric clash with ASF1B. The H4 C terminus (“C-ter”; orange) adopts a helical conformation upon DNAJC9 HBD binding, while it forms a β strand with ASF1B. See also Figure S1.
Figure 3
Molecular basis for recognition of H3.3-H4 by DNAJC9 (A) Multiple sequence alignment of DNAJC9 HBD: H. sapiens (
NP_056005
), M. musculus (
NP_598842
), G. gallus (
NP_001186454
), X. laevis (
NP_001089275
), D. rerio (
NP_001002433
), D. melanogaster (
NP_001262473
), and S. pombe (
NP_594359
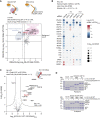
). Under the alignment, red squares indicate residues of DNAJC9 interacting with H3.3-H4; “4A” highlights the multiple mutant disrupting interaction with H3.3-H4; black squares indicate residues of DNAJC9 HBD interacting with MCM2 HBD. (B–D) Enlarged views showing the interaction details between DNAJC9 HBD (magenta) and H3.3-H4 (blue and green, respectively). (E) Effects of DNAJC9 HBD mutants on histone binding using GST pull-downs. 4A1 and 4A corresponding to mutants “E195A E196A E199A A200E” and “Q224A R227A M238A Y242A,” respectively. Quantifications on H3.3 based on replicate experiments (n = 3), expressed as mean ± SEM percentage. The signal of H3.3 in WT lane from the same gel was set to 100%. (F) ITC results of DNAJC9 HBD WT and 4A mutant with histones (n = 3 independent experiments, error bars represent mean ± SD). DNAJC9 HBD WT binds H3.3-H4 with a _K_d of 55.0 ± 19.7 nM and H3.1-H4 with a _K_d of 39.5 ± 4.9 nM. _K_d values represent the mean ± SD of independent measurements (n = 3). No binding was observed between DNAJC9 HBD 4A mutant and H3.3-H4 (n = 3).
Figure 4
DNAJC9 recruits the heat shock molecular chaperone machinery to fold histone H3-H4 substrates (A) DNAJC9 WT, 4A mutant, and control purifications subjected to triple SILAC-based mass spectrometry analysis. Ratios averaged from n = 2 biological replicates. (B) Histone purifications from soluble extracts of cells small interfering RNA (siRNA) depleted for DNAJC9, BAG2, or HSC7C compared with control (CTRL) siRNA and analyzed using label-free mass spectrometry (s0 = 0.5, false discovery rate [FDR] = 0.05, H3.1 n = 5 and H4 n = 4 biological replicates). Bubble plots colors represent Log2 ratios of median-normalized LFQ intensities (siRNA/siCTRL)M.N., and radii represent significance of changes (s0 = 0.5, FDR = 0.05); no imputed values shown. (C) Histone purifications from soluble extracts subjected to label-free mass spectrometry analysis (n = 3 biological replicates, s0 = 0.5, FDR = 0.05). Volcano plots represent differences in median-normalized LFQ intensities (LFQM.N.) with missing values imputed for factors observed three times in either replicate. (D) GST pull-down assays showing H3.3 WT- or H3.3 ED105AA-H4 binding to selected histone chaperones. In (A)–(C), proteins are referred to by human UniProt protein identification code. See also Figures S2 and S3 and Table S1.
Figure 5
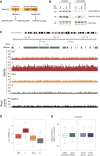
J domain mutation traps DNAJC9 on chromatin genome-wide in a histone-dependent manner (A) DNAJC9 domain map with relevant mutations. (B) Western blots of soluble and chromatin extracts from cells expressing DNAJC9-MYC-FLAG WT, J, or 4AJ mutants compared with control cells. See also Figure S4. (C–E) Quantitative ChIP-seq of cells expressing DNAJC9-MYC-FLAG WT, J, or 4AJ mutants compared with control cells. ChIP-seq reads were quantitated in 10 kb windows with a 5 kb step. Plots represent data averaged from n = 2 biological replicates. (C) Visualization of spike-in normalized ChIP-seq signal in DNAJC9 WT, J, 4AJ, and control samples, quantitated with reference-adjusted reads per million (RRPM), and raw input reads over the region depicted. (D) Boxplots of spike-in normalized DNAJC9 ChIP-seq signal across the genome quantitated with reference-adjusted reads per million (RRPM), Log2(n + 1). Black line, median; whiskers, 1.5 × interquartile range. (E) Boxplots of input corrected signal for DNAJC9 J mutant over gene bodies and intergenic regions (left) or gene bodies parsed to active and inactive genes (right). Black line, median; whiskers, 1.5 × interquartile range.
Figure 6
DNAJC9-directed HSP70 activity facilitates histone supply to and transactions within chromatin (A–D) DNAJC9 WT, mutant, and control purifications from soluble and chromatin extracts subjected to label-free mass spectrometry analysis (n = 6 biological replicates). Proteins are referred to by human UniProt protein identification code. See also Figures S5 and S6 and Table S1. (A) Bubble plots from (left) soluble and (right) chromatin fraction purifications showing enrichment (red) and depletion (blue) in J domain and histone binding mutants (J and 4A, respectively) compared with WT. Ratios calculated from bait-normalized LFQ intensities (LFQB.N.). Data analysis steps are detailed in Figures S5 and S6. (B) Left: Euclidean clustering analysis of soluble fraction LFQB.N. intensities for factors identified in at least six of six experiments for WT, J, or 4A mutants and additionally significantly enriched in at least one set over the control purifications (S0 = 2, FDR = 0.01). Region of interest highlighted (black box) and magnified (right) to show trends of enrichment and depletion of histones, ribosomal proteins, HSP70s and other factors. (C) Western blots of soluble extracts from cells expressing DNAJC9 WT, J, or 4A mutants and control cells showing the accumulation of soluble histones in cells expressing the DNAJC9 J mutant. (D) STRING-db network of factors most significantly enriched in DNAJC9 WT purifications and overlaid ratios of enrichment/depletion in J and 4A mutants. Sixteen of 22 nodes with Log2 (LFQ WT/C)B.N. > 1.8 were connected to each other at a STRING confidence level of 0.6; red edges represent nodes connected with experimental evidence.
Figure 7
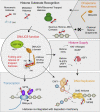
DNAJC9 links heat shock biology to the histone chaperone network DNAJC9 binds histone substrates that cannot engage other histone chaperones because of being monomeric, misfolded, or engaged in spurious interactions with RNA/DNA. DNAJC9 recruits HSP70-type enzymes through its J domain to fold and release of histones substrates with ATP-derived energy. DNAJC9-bound histones can enter the histone chaperone supply chain upstream of HAT1 for their eventual delivery to chromatin by ASF1. Alternatively, DNAJC9-bound histone dimers bypass ASF1 and engage with histone deposition chaperones during DNA replication and transcription.
Comment in
- An energetic meet-and-greet: Molecular chaperones in the histone supply and deposition pathways.
Dreyer J, Mattiroli F. Dreyer J, et al. Mol Cell. 2021 Jun 17;81(12):2499-2501. doi: 10.1016/j.molcel.2021.05.012. Mol Cell. 2021. PMID: 34143966
Similar articles
- DNAJC9 prevents CENP-A mislocalization and chromosomal instability by maintaining the fidelity of histone supply chains.
Balachandra V, Shrestha RL, Hammond CM, Lin S, Hendriks IA, Sethi SC, Chen L, Sevilla S, Caplen NJ, Chari R, Karpova TS, McKinnon K, Todd MA, Koparde V, Cheng KC, Nielsen ML, Groth A, Basrai MA. Balachandra V, et al. EMBO J. 2024 Jun;43(11):2166-2197. doi: 10.1038/s44318-024-00093-6. Epub 2024 Apr 10. EMBO J. 2024. PMID: 38600242 Free PMC article. - A unique binding mode enables MCM2 to chaperone histones H3-H4 at replication forks.
Huang H, Strømme CB, Saredi G, Hödl M, Strandsby A, González-Aguilera C, Chen S, Groth A, Patel DJ. Huang H, et al. Nat Struct Mol Biol. 2015 Aug;22(8):618-26. doi: 10.1038/nsmb.3055. Epub 2015 Jul 13. Nat Struct Mol Biol. 2015. PMID: 26167883 Free PMC article. - Structural insight into how the human helicase subunit MCM2 may act as a histone chaperone together with ASF1 at the replication fork.
Richet N, Liu D, Legrand P, Velours C, Corpet A, Gaubert A, Bakail M, Moal-Raisin G, Guerois R, Compper C, Besle A, Guichard B, Almouzni G, Ochsenbein F. Richet N, et al. Nucleic Acids Res. 2015 Feb 18;43(3):1905-17. doi: 10.1093/nar/gkv021. Epub 2015 Jan 23. Nucleic Acids Res. 2015. PMID: 25618846 Free PMC article. - The chaperone-histone partnership: for the greater good of histone traffic and chromatin plasticity.
Hondele M, Ladurner AG. Hondele M, et al. Curr Opin Struct Biol. 2011 Dec;21(6):698-708. doi: 10.1016/j.sbi.2011.10.003. Epub 2011 Nov 3. Curr Opin Struct Biol. 2011. PMID: 22054910 Review. - All roads lead to chromatin: multiple pathways for histone deposition.
Li Q, Burgess R, Zhang Z. Li Q, et al. Biochim Biophys Acta. 2013 Mar-Apr;1819(3-4):238-46. Biochim Biophys Acta. 2013. PMID: 24459726 Review.
Cited by
- Interaction of client-the scaffold on which FeS clusters are build-with J-domain protein Hsc20 and its evolving Hsp70 partners.
Marszalek J, Craig EA. Marszalek J, et al. Front Mol Biosci. 2022 Oct 12;9:1034453. doi: 10.3389/fmolb.2022.1034453. eCollection 2022. Front Mol Biosci. 2022. PMID: 36310602 Free PMC article. Review. - Identifying the genes impacted by cell proliferation in proteomics and transcriptomics studies.
Locard-Paulet M, Palasca O, Jensen LJ. Locard-Paulet M, et al. PLoS Comput Biol. 2022 Oct 6;18(10):e1010604. doi: 10.1371/journal.pcbi.1010604. eCollection 2022 Oct. PLoS Comput Biol. 2022. PMID: 36201535 Free PMC article. - The Role of Endoplasmic Reticulum Stress on Reducing Recombinant Protein Production in Mammalian Cells.
Splichal RC, Chen K, Walton SP, Chan C. Splichal RC, et al. Biochem Eng J. 2024 Oct;210:109434. doi: 10.1016/j.bej.2024.109434. Epub 2024 Jul 20. Biochem Eng J. 2024. PMID: 39220803 - DNAJC9 prevents CENP-A mislocalization and chromosomal instability by maintaining the fidelity of histone supply chains.
Balachandra V, Shrestha RL, Hammond CM, Lin S, Hendriks IA, Sethi SC, Chen L, Sevilla S, Caplen NJ, Chari R, Karpova TS, McKinnon K, Todd MA, Koparde V, Cheng KC, Nielsen ML, Groth A, Basrai MA. Balachandra V, et al. EMBO J. 2024 Jun;43(11):2166-2197. doi: 10.1038/s44318-024-00093-6. Epub 2024 Apr 10. EMBO J. 2024. PMID: 38600242 Free PMC article. - DAXX adds a de novo H3.3K9me3 deposition pathway to the histone chaperone network.
Carraro M, Hendriks IA, Hammond CM, Solis-Mezarino V, Völker-Albert M, Elsborg JD, Weisser MB, Spanos C, Montoya G, Rappsilber J, Imhof A, Nielsen ML, Groth A. Carraro M, et al. Mol Cell. 2023 Apr 6;83(7):1075-1092.e9. doi: 10.1016/j.molcel.2023.02.009. Epub 2023 Mar 2. Mol Cell. 2023. PMID: 36868228 Free PMC article.
References
- Alekseev O.M., Widgren E.E., Richardson R.T., O’Rand M.G. Association of NASP with HSP90 in mouse spermatogenic cells: stimulation of ATPase activity and transport of linker histones into nuclei. J. Biol. Chem. 2005;280:2904–2911. - PubMed
- Allis C.D., Jenuwein T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016;17:487–500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous