Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine - A Comprehensive Review - PubMed (original) (raw)
Review
Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine - A Comprehensive Review
Dane Kim et al. Front Immunol. 2021.
Abstract
A unique subpopulation of mesenchymal stem cells (MSCs) has been isolated and characterized from human gingival tissues (GMSCs). Similar to MSCs derived from other sources of tissues, e.g. bone marrow, adipose or umbilical cord, GMSCs also possess multipotent differentiation capacities and potent immunomodulatory effects on both innate and adaptive immune cells through the secretion of various types of bioactive factors with immunosuppressive and anti-inflammatory functions. Uniquely, GMSCs are highly proliferative and have the propensity to differentiate into neural cell lineages due to the neural crest-origin. These properties have endowed GMSCs with potent regenerative and therapeutic potentials in various preclinical models of human disorders, particularly, some inflammatory and autoimmune diseases, skin diseases, oral and maxillofacial disorders, and peripheral nerve injuries. All types of cells release extracellular vesicles (EVs), including exosomes, that play critical roles in cell-cell communication through their cargos containing a variety of bioactive molecules, such as proteins, nucleic acids, and lipids. Like EVs released by other sources of MSCs, GMSC-derived EVs have been shown to possess similar biological functions and therapeutic effects on several preclinical diseases models as GMSCs, thus representing a promising cell-free platform for regenerative therapy. Taken together, due to the easily accessibility and less morbidity of harvesting gingival tissues as well as the potent immunomodulatory and anti-inflammatory functions, GMSCs represent a unique source of MSCs of a neural crest-origin for potential application in tissue engineering and regenerative therapy.
Keywords: anti-inflammation; gingiva-derived mesenchymal stem cells; immunomodulation; neural crest; regenerative therapy.
Copyright © 2021 Kim, Lee, Xu, Zhang and Le.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
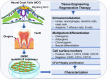
Figure 1
Isolation and characterization of mesenchymal stem cells derived from gingival tissues of neural crest origin. A unique subpopulation of mesenchymal stem cells can be isolated from neural crest-derived gingival tissues (GMSC), thus representing a reservoir of neural crest-derived MSCs. CFU-F, colony forming unit-fibroblast. Portions of this figure were made using templates from SMART SERVIER MEDICAL ART (
) and Vecteezy (
).
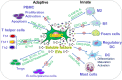
Figure 2
Immunomodulatory effects of GMSCs on both innate and adaptive immune cells. PBMC, peripheral blood mononuclear cells; M2, M2 macrophages; M1, M1 macrophages; DC, dendritic cells; IDO, indoleamine 2,3-dioxygenase; COX2, cyclooxygenase-2; PGE2, prostaglandin 2; FasL, Fas ligand; EVs, extracellular vesicles; IL-10, interleukin-10; IL-6, interleukin-6. “⊕” means blocking or inhibiting. Portions of this figure were made using templates from SMART SERVIER MEDICAL ART (
).
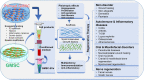
Figure 3
Application of GMSCs and their cell-free products in regenerative therapy. Naïve or primed GMSCs, their derivative conditioned medium or extracellular vesicles (EVs) can be administered alone or in combination with certain scaffold, growth factor or small molecules. Due to the immunomodulatory/anti-inflammatory and pleiotropic effects, GMSCs and their derivative cell-free products exert potent regenerative and therapeutic potentials in a variety of preclinical models of human disorders. Portions of this figure were made using templates from SMART SERVIER MEDICAL ART (
).
Similar articles
- A Narrative Review: Gingival Stem Cells as a Limitless Reservoir for Regenerative Medicine.
Fonticoli L, Della Rocca Y, Rajan TS, Murmura G, Trubiani O, Oliva S, Pizzicannella J, Marconi GD, Diomede F. Fonticoli L, et al. Int J Mol Sci. 2022 Apr 8;23(8):4135. doi: 10.3390/ijms23084135. Int J Mol Sci. 2022. PMID: 35456951 Free PMC article. Review. - Characteristics and Tissue Regeneration Properties of Gingiva-Derived Mesenchymal Stem Cells.
Zhao N, Wu Z, Qin L, Guo Z, Li D. Zhao N, et al. Crit Rev Eukaryot Gene Expr. 2015;25(2):135-44. doi: 10.1615/critreveukaryotgeneexpr.2015012539. Crit Rev Eukaryot Gene Expr. 2015. PMID: 26080607 Review. - Negative effects of a high tumour necrosis factor-α concentration on human gingival mesenchymal stem cell trophism: the use of natural compounds as modulatory agents.
Giacomelli C, Natali L, Nisi M, De Leo M, Daniele S, Costa B, Graziani F, Gabriele M, Braca A, Trincavelli ML, Martini C. Giacomelli C, et al. Stem Cell Res Ther. 2018 May 11;9(1):135. doi: 10.1186/s13287-018-0880-7. Stem Cell Res Ther. 2018. PMID: 29751776 Free PMC article. - Gingiva-derived Mesenchymal Stem Cells and Their Potential Applications in Oral and Maxillofacial Diseases.
Gao X, Cao Z. Gao X, et al. Curr Stem Cell Res Ther. 2020;15(1):43-53. doi: 10.2174/1574888X14666191107100311. Curr Stem Cell Res Ther. 2020. PMID: 31702517 - Stemness Potency of Human Gingival Cells-Application in Anticancer Therapies and Clinical Trials.
Stefańska K, Mehr K, Wieczorkiewicz M, Kulus M, Angelova Volponi A, Shibli JA, Mozdziak P, Skowroński MT, Antosik P, Jaśkowski JM, Piotrowska-Kempisty H, Kempisty B, Dyszkiewicz-Konwińska M. Stefańska K, et al. Cells. 2020 Aug 18;9(8):1916. doi: 10.3390/cells9081916. Cells. 2020. PMID: 32824702 Free PMC article. Review.
Cited by
- Resveratrol May Reduce the Degree of Periodontitis by Regulating ERK Pathway in Gingival-Derived MSCs.
Jiang H, Ni J, Hu L, Xiang Z, Zeng J, Shi J, Chen Q, Li W. Jiang H, et al. Int J Mol Sci. 2023 Jul 10;24(14):11294. doi: 10.3390/ijms241411294. Int J Mol Sci. 2023. PMID: 37511053 Free PMC article. - Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications.
Li P, Ou Q, Shi S, Shao C. Li P, et al. Cell Mol Immunol. 2023 Jun;20(6):558-569. doi: 10.1038/s41423-023-00998-y. Epub 2023 Mar 27. Cell Mol Immunol. 2023. PMID: 36973490 Free PMC article. Review. - Gingiva-derived Stromal Cells Isolated from Cats Affected with Tooth Resorption Exhibit Increased Apoptosis, Inflammation, and Oxidative Stress while Experiencing Deteriorated Expansion and Anti-Oxidative Defense.
Soltero-Rivera M, Groborz S, Janeczek M, Kornicka J, Wierzgon M, Arzi B, Marycz K. Soltero-Rivera M, et al. Stem Cell Rev Rep. 2023 Jul;19(5):1507-1523. doi: 10.1007/s12015-023-10537-x. Epub 2023 Apr 11. Stem Cell Rev Rep. 2023. PMID: 37039946 - MSCs' conditioned media cytokine and growth factor profiles and their impact on macrophage polarization.
Peshkova M, Korneev A, Suleimanov S, Vlasova II, Svistunov A, Kosheleva N, Timashev P. Peshkova M, et al. Stem Cell Res Ther. 2023 May 25;14(1):142. doi: 10.1186/s13287-023-03381-w. Stem Cell Res Ther. 2023. PMID: 37231519 Free PMC article. - Exosomes derived from odontogenic stem cells: Its role in the dentin-pulp complex.
Zou J, Xia H, Jiang Q, Su Z, Wen S, Liang Z, Ouyang Y, Liu J, Zhang Z, Chen D, Yang L, Guo L. Zou J, et al. Regen Ther. 2023 Jun 22;24:135-146. doi: 10.1016/j.reth.2023.05.008. eCollection 2023 Dec. Regen Ther. 2023. PMID: 37415682 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources