Visualizing the In Vivo Dynamics of Anti- Leishmania Immunity: Discoveries and Challenges - PubMed (original) (raw)
Review
Visualizing the In Vivo Dynamics of Anti- Leishmania Immunity: Discoveries and Challenges
Romaniya Zayats et al. Front Immunol. 2021.
Abstract
Intravital microscopy, such as 2-photon microscopy, is now a mainstay in immunological research to visually characterize immune cell dynamics during homeostasis and pathogen infections. This approach has been especially beneficial in describing the complex process of host immune responses to parasitic infections in vivo, such as Leishmania. Human-parasite co-evolution has endowed parasites with multiple strategies to subvert host immunity in order to establish chronic infections and ensure human-to-human transmission. While much focus has been placed on viral and bacterial infections, intravital microscopy studies during parasitic infections have been comparatively sparse. In this review, we will discuss how in vivo microscopy has provided important insights into the generation of innate and adaptive immunity in various organs during parasitic infections, with a primary focus on Leishmania. We highlight how microscopy-based approaches may be key to providing mechanistic insights into Leishmania persistence in vivo and to devise strategies for better parasite control.
Keywords: Leishmania infection; T cells; ear skin imaging; fluorescent reporters; liver imaging; macrophages; two-photon intravital microscopy.
Copyright © 2021 Zayats, Uzonna and Murooka.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Figure 1
2P-microscopy is the gold standard for intravital imaging. (A) Jablonski diagram, illustrating the principles of excitation with one Ultraviolet (UV) photon and two Infrared (IR) photons. (B) Spatial confinement of signal generation. 1-photon excitation generates visible (green) signal in the entire cone of fluorescence, while 2-photon excitation generates a signal only at the focal spot. (C) 1-photon excitation microscopy of the skin leads to a low light penetration, which becomes scattered by melanocytes and erythrocytes, while 2-photon excitation leads to a deeper penetration will less light scattering.
Figure 2
In vivo microscopy of the liver in _Leishmania_-infected mice (A) Graphical illustration of a mouse preparation for short-term liver imaging. Liver is externalized, placed onto the silicone bed, and covered with a custom metal cover slide. (B) Graphical illustration of a mouse preparation for long-term liver imaging. A titanium ring is surgically inserted into the mouse abdomen and connected to a custom stabilizing mechanism. (C) Granuloma formation in the liver at 14 vs 25 days post infection with Leishmania donovani. CD8+ T cells are recruited to the granuloma irrespective of antigen specificity, but antigen specific CD8+ T cells are retained at 25 days. (D) Pathogenic and protective roles of B cells in Leishmania immunity. Leishmania donovani can trigger endosomal TLR stimulation, induce hypergammaglobulinemia and increase type I interferons (IFN-I) expression (left panel). IgGs from B cells facilitate opsonization of Leishmania major parasites by DCs via Fc receptors to drive effector Th1 activation (right panel).
Figure 3
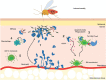
Acute response to Leishmania infection by a sandfly bite. (1) Neutrophils (dark blue) are recruited from the blood to the site of infection, undergo NETosis, and phagocytose the promastigotes. (2) Infected neutrophils recruit DCs (green) by producing CCL3, which subsequently engulf the apoptotic bodies of infected neutrophils and (3) lose their ability to effectively activate Th1 response (light blue). (4) Macrophages (dark green) become infected by the parasites released by the dying neutrophils. (5) CD11c+ monocytes (green) are highly permissive to parasite replication and further promote infection.
Figure 4
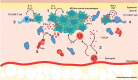
Establishment of chronic Leishmania infection. (1) M2-like dermal macrophages (dark green) harbor parasites. (2) Eosinophils (red) are recruited to the site of infection via CCL24 and produce IL-4 to maintain the M2-like dermal macrophages. (3) Th2 CD4+ T cells (light blue) exacerbate disease progression, while (4) Th1 CD4+ T cells (dark blue) stimulate macrophages to improve leishmanicidal activity, mediated primarily by IFN_γ_ production. At this stage of infection, Th1 CD4+ T cells tend to surround but do not enter the lesion. (5) Regulatory T cells (red) are recruited to the lesion site and can suppress Th1 responses, possibly through IL-10 production.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical