Inhibiting endocytosis in CGRP+ nociceptors attenuates inflammatory pain-like behavior - PubMed (original) (raw)
Inhibiting endocytosis in CGRP+ nociceptors attenuates inflammatory pain-like behavior
Rasheen Powell et al. Nat Commun. 2021.
Abstract
The advantage of locally applied anesthetics is that they are not associated with the many adverse effects, including addiction liability, of systemically administered analgesics. This therapeutic approach has two inherent pitfalls: specificity and a short duration of action. Here, we identified nociceptor endocytosis as a promising target for local, specific, and long-lasting treatment of inflammatory pain. We observed preferential expression of AP2α2, an α-subunit isoform of the AP2 complex, within CGRP+/IB4- nociceptors in rodents and in CGRP+ dorsal root ganglion neurons from a human donor. We utilized genetic and pharmacological approaches to inhibit nociceptor endocytosis demonstrating its role in the development and maintenance of acute and chronic inflammatory pain. One-time injection of an AP2 inhibitor peptide significantly reduced acute and chronic pain-like behaviors and provided prolonged analgesia. We evidenced sexually dimorphic recovery responses to this pharmacological approach highlighting the importance of sex differences in pain development and response to analgesics.
© 2021. The Author(s).
Conflict of interest statement
A. Bhattacharjee is the co-founder of Channavix Inc., a company developing non-opioids drugs for pain. A patent has been filed on the use of lipidated peptidomimetics targeting endocytosis to treat pain. All other authors declare no competing interests.
Figures
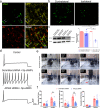
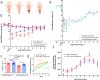
Fig. 1. AP2α2 is expressed in IB4-, CGRP+ nociceptors, and in vivo AP2α2 knockdown attenuates nocifensive behaviors.
a Representative immunofluorescent image showing expression patterns of AP2α2 and CGRP. IB4 reactivity was used to delineate between peptidergic and non-peptidergic DRG neurons. AP2α2 is preferentially expressed in small- and medium-sized CGRP+ DRG neurons but not in IB4+ neurons. Arrows highlight strong co-localization of CGRP and AP2α2. This experiment was repeated independently 3 times and similar results were obtained. b AP2α2 immunoreactivity in the ipsilateral DRG after in vivo AP2α2 knockdown compared to contralateral DRG taken from the same animal, seven days after knockdown. This experiment was repeated independently 3 times, and similar results were obtained. c [Left] Representative Western blot showing extent of AP2α2 knockdown. Paired contralateral and ipsilateral samples are taken from the same animal. [Right] Densitometry analysis of Western blots. Samples were collected from biologically independent animals (n = 3 for scrambled shRNA and n = 3 for AP2α2 shRNA). Animals were sacrificed seven days after knockdown. Data is presented as mean pixel density ± S.E.M. Significance determined by one-way ANOVA with Holms-Sidak correction p < 0.05; *p < 0.01; **. Scrambled shRNA Contra vs AP2α2 shRNA Ipsi _p_-value = 0.0011. Scrambled shRNA Ipsi vs AP2α2 Ipsi shRNA _p_-value = 0.0025. AP2α2 shRNA Contra vs AP2α2 shRNA Ipsi _p_-value = 0.0011. d Representative traces from dissociated adult DRG neurons recorded under varying conditions: [Top] Control conditions, [Middle] DRG neurons transfected with Scrambled shRNAs following 30-min exposure to Adenosine-3’, 5’- cyclic monophosphate, Sp- isomer (Sp-cAMPs), [Bottom] DRG neurons transfected with AP2α2 shRNAs during PKA stimulatory conditions. IB4- were selectively recorded as determined by absence of fluorescent after incubation with an IB4-alexa fluor 488 conjugate. e Representative images depicting pain-like behaviors in C57BL/6 mice 2 min [left], 20 min [middle], and 60 min [right] post-formalin injection. Red arrow highlights use of inflamed ipsilateral paw. f Summarized nocifensive behaviors from C57BL/6 mice following injection with 5% formalin. Phase 1; 0–10 min, phase 2; 11–60 min post injection (scrambled shRNA group n = 6; AP2α2 group n = 6). Data is pooled males and females and is presented as cumulative means ± S.E.M. Significance determined using repeated measures 2-way ANOVA with Bonferroni correction p < 0.05; *p < 0.01; **. Cumulative # of licks: Phase 2 Scrambled shRNA vs AP2α2 shRNA _p_-value = 0.0313. Cumulative Paw Elevation: Phase 2 Scrambled shRNA vs AP2α2 shRNA _p_-value = 0.0026.
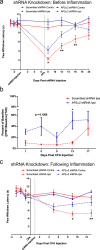
Fig. 2. AP2α2 knockdown disrupts the development and maintenance of thermal sensitivity in chronic inflammatory pain.
a Thermal sensitivity of animals in the CFA pain model with a pre-inflammatory knockdown paradigm for scrambled (n = 11) and AP2α2 (n = 12) shRNA groups. Data is pooled males and females and is represented as mean paw withdrawal latency (PWL) ± S.E.M. Significance determined using repeated measures 2-way ANOVA with Bonferroni correction p < 0.05; *p < 0.01; ** Day 8: Ipsilateral Scrambled shRNA vs Ipsilateral AP2α2 shRNA _p_-value = 0.0398. Day 12: Ipsilateral scrambled shRNA vs Ipsilateral AP2α2 shRNA _p_-value = 0.0048. Day 16: Ipsilateral scrambled shRNA vs ipsilateral AP2α2 shRNA _p_-value = 0.0013. b von Frey withdrawal threshold of ipsilateral hind paw in a chronic inflammatory pain model where knockdown occurred before inflammation was initiated. Data for the scrambled (n = 8) and AP2α2 (n = 8) shRNA groups is pooled, males and females, and is represented as mean percentage of baseline ± S.E.M. Significance determined using repeated measures 2-way ANOVA with Bonferroni correction p < 0.05; *. Day 13: Scrambled shRNA vs AP2α2 shRNA _p_-value = 0.0315. Contralateral PWT can be found in Supplementary Fig. 2. c Thermal sensitivity of animals injected with scrambled (n = 8) and AP2α2 (n = 8) shRNAs following establishment of inflammation. Data is pooled males and females and is represented as mean PWL ± S.E.M. Significance determined using repeated measures 2-way ANOVA with Bonferroni correction p < 0.05; *p < 0.01; **. Day 9: Ipsilateral scrambled shRNA vs ipsilateral AP2α2 shRNA _p_-value = 0.0251. Day 13: Ipsilateral scrambled shRNA vs Ipsilateral AP2α2 shRNA _p_-value = 0.0064.
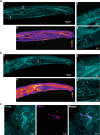
Fig. 3. Lipidated peptides infiltrate peripheral neuronal afferents.
a Tilescan immunofluorescent image of a mouse hind paw following injection of an antigenic lipidated-HA peptide into the hind paw of a naïve C57BL/6 mouse under control conditions. The HA-peptide allowed for immunofluorescent visualization of lipidated peptide distribution following injection. This experiment was repeated, independently, twice with similar results. a4 Heatmap analysis of immunoreactivity. a1 The lipidated-HA peptide preferentially partitioned to the dermis, within lipid dense areas. a2 Insert depicting HA immunoreactivity in peripheral afferents within the dermis. a3 Insert depicting immunoreactivity in peripheral afferents in muscle tissue. While afferents exhibited immunolabeling, muscle cells did not. b Injection of an antigenic lipidated peptidomimetic into the hind paw of a naïve C57BL/6 mouse under CFA-induced inflammation. This experiment was repeated, independently, twice with similar results. b4 Heatmap analysis depicted greater retention of peptide within inflamed tissues. b1 Again, lipidated-HA peptide preferentially partitioned to the dermis, specifically, lipid dense areas. b2 Insert depicting immunoreactivity in peripheral afferents in the dermis. b3 Insert depicting immunoreactivity in peripheral afferents and in muscle tissue. c Immunofluorescent image depicting localization of the membrane bound HA-peptide and the cytoplasmic pan-neuronal marker PGP9.5. This experiment was repeated, independently, twice with similar results. [Left] HA-peptide alone. Arrows highlight the contour of the fiber. [Middle] PGP9.5 alone. Arrows illustrate the contour of the neuron afferent observed with the HA-peptide. [Right] Merged image. Arrows show that the HA-peptide and PGP9.5 immunoreactivity follow similar contours.
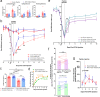
Fig. 4. Pharmacological inhibition of peripheral endocytosis by a lipidated AP2 inhibitor peptide attenuated pain-like behaviors during acute and chronic inflammation.
a Summarized nocifensive behaviors from C57BL/6 mice following injection with 5% formalin. The AP2 inhibitor peptide inhibitor was locally injected into the hind paw 24 h before formalin injection. Phase 1; 0–10 min, phase 2; 15–60 min post-injection (Scrambled peptide group n = 6; AP2 inhibitor peptide group n = 6). Data is pooled male and female mice and is presented as mean ± S.E.M. Significance determined using repeated measures 2-way ANOVA with Bonferroni correction p < 0.05; *Cumulative # of Licks: Phase 2 Scrambled peptide vs AP2 inhibitor peptide _p_-value = 0.0040. b Thermal sensitivity of animals that received either the scrambled peptide (n = 16) or the AP2 inhibitor peptide (n = 16) during established CFA-induced inflammatory pain. Each group was injected with the respective peptide 24 h after CFA. Data is pooled male and female animals and is represented as mean PWL ± S.E.M. Significance determined using repeated measures 2-way ANOVA with Bonferroni correction p < 0.01; **p < 0.005; ***. Day 2: Ipsilateral scrambled peptide vs Ipsilateral AP2 inhibitor peptide _p_-value = 0.0027. Day 3: Ipsilateral scrambled peptide vs Ipsilateral AP2 inhibitor peptide _p_-value < 0.0001. Day 5: Ipsilateral scrambled peptide vs Ipsilateral AP2 inhibitor peptide _p_-value = 0.0057. c Recovery area under the curve (A.U.C.; bounded by days 1–13)) quantification for all animals under experimental conditions displayed in (B; Days 1–13); scrambled peptide (n = 16) and AP2 inhibitor peptide (n = 16). Data shown is pooled male and female animals and is represented as the mean A.U.C. (in arbitrary units; Arb.u.) ± S.E.M. Statistical significance was determined using one-way ANOVA with Holms-Sidak correction p < 0.05; *p < 0.01; **p < 0.005; ***p < 0.001; ****. Contralateral scrambled peptide vs Ipsilateral scrambled peptide _p_-value < 0.0001. Contralateral Scrambled peptide vs ipsilateral AP2 Inhibitor peptide _p_-value = 0.0006. Contralateral AP2 inhibitor peptide vs Ipsilateral Scrambled peptide p < 0.0001. Contralateral AP2 inhibitor peptide vs Ipsilateral AP2 inhibitor peptide p < 0.0001. Ipsilateral scrambled peptide vs ipsilateral AP2 inhibitor peptide _p_-value < 0.0001. Pharmacological inhibition of endocytosis generated a significant increase in A.U.C. for the ipsilateral paw. d Recovery curve (bounded by days 1–13) fit to an exponential decay equation. Fitting the recovery curves from b reveals that inhibition of endocytosis accelerated the rate of recovery indicated by a decrease in tau. e Graph depicting the thermal sensitivity of the ipsilateral paw in male (n = 8; green) and female (n = 8; purple) animals that received the AP2 inhibitor peptide. Gray line represents mean AP2 inhibitor peptide ipsilateral paw withdraw threshold from b. Boxed area corresponds to data point of interest. Data is represented as the mean paw withdrawal ± S.E.M. Significance was determined using a 3-way ANOVA with Fishers Least Significant Difference Post-hoc test p < 0.5; *p < 0.01; **. Male Ipsilateral AP2 peptide vs Female AP2 inhibitor peptide _p_-value = 0.0069. f Percent of hind paw weight borne on the ipsilateral paw 24 h following CFA injection and 48 h following peptide injection. [Top] Change in percent of hind paw weight borne on the ipsilateral hind paw of animals that received the scrambled peptide (n = 8). [Bottom] Change in percent of hind paw weight borne on the ipsilateral hind paw of animals that received the AP2 inhibitor peptide (n = 8). Injection of the AP2 inhibitor peptide was sufficient in increasing weight bearing efficiency of the ipsilateral hind paw compared to 24 h following CFA injection. Injection of the scrambled peptide did not accelerate weight bearing efficiency of the ipsilateral hind paw. Data is pooled male and female mice and is represented as mean weight borne on the ipsilateral hind paw. Significance determined using a one-tailed paired Student’s t test p < 0.05; *. 24 hours post-CFA vs 48 hours post-AP2 peptide _p_-value = 0.0483. g Ipsilateral von Frey withdraw threshold of animals in either the scrambled peptide (n = 11) or the AP2 inhibitor peptide (n = 11) groups following establishment of CFA-induced inflammatory pain. Data is pooled male and female mice and is represented as mean PWT (as a percentage of baseline PWT) ± S.E.M. Significance determined using repeated measures 2-way ANOVA with Bonferroni correction p < 0.05; * Contralateral PWT can be found in Supplementary Fig. 4.
Fig. 5. Pharmacological inhibition of peripheral endocytosis by a lipidated AP2 inhibitor peptide attenuated thermal sensitivity in a post-operative pain model.
a Schematic depicting injection protocol for lipidated AP2 inhibitor peptide. b Graph depicting thermal sensitivity in rats following plantar muscle incision and injection with scrambled peptide (n = 14) or AP2 inhibitor peptide (n = 18). Data shown is pooled males and females. Data is represented as mean PWL ± S.E.M. Significance determined using repeated measures 2-way ANOVA with Bonferroni correction p < 0.05; *p < 0.01; **. Ipsilateral scrambled peptide vs Ipsilateral AP2 peptide: Day 1 _p_-value = 0.0055, Day 3 _p_-value = 0.0048, Day 4 _p_-value = 0.0015, Day 5 _p_-value = 0.0082. c Total area under the curve (A.U.C.) quantification for all rats under experimental conditions displayed in b; scrambled peptide (n = 14) and AP2 inhibitor peptide (n = 18). Pharmacological inhibition of endocytosis generated a significant increase in A.U.C. for the ipsilateral paw. Data shown is pooled males and females. Data is represented as the mean A.U.C. (in arbitrary units; Arb.u.) ± S.E.M. Statistical significance was determined using one-way ANOVA with Holms-Sidak correction p < 0.01; **p < 0.005; ***p < 0.001; ****. Contralateral scrambled peptide vs ipsilateral scrambled peptide _p_-value < 0.0001. Contralateral scrambled peptide vs ipsilateral AP2 inhibitor peptide _p_-value < 0.0001. Contralateral AP2 inhibitor peptide vs ipsilateral scrambled peptide p < 0.0001. Contralateral AP2 inhibitor peptide vs ipsilateral AP2 inhibitor peptide p < 0.0001. Ipsilateral scrambled peptide vs ipsilateral AP2 inhibitor peptide _p_-value = 0.0004. d Recovery curve (Days 1–9) fit to an exponential decay equation. Fitting the recovery curves from b reveals that inhibition of endocytosis accelerated the rate of recovery as indicated by a decrease in tau. e Graph depicting the thermal sensitivity of the ipsilateral paw in male (n = 7; green) and female (n = 7; purple) animals that received the scrambled peptide. Gray line represents mean scrambled peptide ipsilateral paw withdraw threshold from b. Boxed area corresponds to data point of interest. Data is represented as the mean paw withdrawal ± S.E.M. Boxed area corresponds to 24 h post incision. Significance was determined using a 3-way ANOVA with Fishers Least Significant Difference Post-hoc test p < 0.5; *p < 0.01; **. Male contralateral scrambled peptide vs female contralateral scrambled peptide _p_-value = 0.0160. f Ipsilateral von Frey withdraw thresholds of animals in either the scrambled peptide (n = 8) or the AP2 inhibitor peptide (n = 8) post-incision. Data is pooled from male and female rats and is represented as mean PWT (as a percentage of baseline PWT) ± S.E.M. Significance determined using repeated measures 2-way ANOVA with Bonferroni correction. Contralateral PWT can be found in Supplementary Fig. 5.
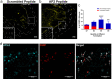
Fig. 6. Local inhibition of endocytosis in peripheral nociceptors potentiated CGRP immunoreactivity in the superficial layers of the dermis and Ap2α2 is differentially distributed in CGRP+ human DRG neurons.
a Representative image showing CGRP immunoreactivity in an uninflamed hind paw injected with the scrambled peptide. Typically, CGRP immunoreactivity terminates in the proximal stratum granulosum (SG). b Representative image showing CGRP immunoreactivity in an uninflamed hind paw injected with the AP2 inhibitor peptide. White arrows_:_ peripheral nerve fibers exhibited robust CGRP immunoreactivity in the very distal layers of the SG and some CGRP immunoreactive fibers could be seen in very superficial stratum corneum (SC) layer. Yellow arrows_;_ peripheral nerve fibers displaying CGRP immunoreactivity in superficial layers of the SC. (Inserts) Magnified sections illustrating SG quadrants. c Quantification of CGRP+ afferent termination in each SG quadrant (Q1, Q2, Q3, and Q4; n = 3). Significance was determined using a multiple t-test using Holms-Sidak correction with p < 0.05; *p < 0.01;**p < 0.005;***p < 0.001;****. Quartile 3 _p_-value = 0.0004. Quartile 4 _p_-value = 0.0076. Data is presented as mean ± S.E.M. d Representative immunofluorescent image showing expression patterns of AP2α2 (left) and CGRP (middle) in hDRGs. AP2α2 is differential expressed in CGRP+ DRG neurons. Arrows highlight strong co-localization of CGRP and AP2α2. SG Stratum Granulosum, SS Stratum Spinosum, SC Stratum Corneum.
Similar articles
- Sensitization of primary afferent nociceptors induced by intradermal capsaicin involves the peripheral release of calcitonin gene-related Peptide driven by dorsal root reflexes.
Li D, Ren Y, Xu X, Zou X, Fang L, Lin Q. Li D, et al. J Pain. 2008 Dec;9(12):1155-68. doi: 10.1016/j.jpain.2008.06.011. Epub 2008 Aug 13. J Pain. 2008. PMID: 18701354 Free PMC article. - Characteristics of sensory dorsal root ganglia neurons innervating the lumbar vertebral body in rats.
Ohtori S, Inoue G, Koshi T, Ito T, Yamashita M, Yamauchi K, Suzuki M, Doya H, Moriya H, Takahashi Y, Takahashi K. Ohtori S, et al. J Pain. 2007 Jun;8(6):483-8. doi: 10.1016/j.jpain.2007.01.004. Epub 2007 Mar 26. J Pain. 2007. PMID: 17382597 - CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons.
Qin X, Wan Y, Wang X. Qin X, et al. J Neurosci Res. 2005 Oct 1;82(1):51-62. doi: 10.1002/jnr.20612. J Neurosci Res. 2005. PMID: 16047385 - Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF.
Giniatullin R, Nistri A, Fabbretti E. Giniatullin R, et al. Mol Neurobiol. 2008 Feb;37(1):83-90. doi: 10.1007/s12035-008-8020-5. Epub 2008 May 6. Mol Neurobiol. 2008. PMID: 18459072 Review. - Pain pharmacology in migraine: focus on CGRP and CGRP receptors.
Benemei S, Nicoletti P, Capone JA, Geppetti P. Benemei S, et al. Neurol Sci. 2007 May;28 Suppl 2:S89-93. doi: 10.1007/s10072-007-0757-5. Neurol Sci. 2007. PMID: 17508187 Review.
Cited by
- Role of Clathrin and Dynamin in Clathrin Mediated Endocytosis/Synaptic Vesicle Recycling and Implications in Neurological Diseases.
Prichard KL, O'Brien NS, Murcia SR, Baker JR, McCluskey A. Prichard KL, et al. Front Cell Neurosci. 2022 Jan 18;15:754110. doi: 10.3389/fncel.2021.754110. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35115907 Free PMC article. Review. - Osteoarthritis Pain.
Yu H, Huang T, Lu WW, Tong L, Chen D. Yu H, et al. Int J Mol Sci. 2022 Apr 22;23(9):4642. doi: 10.3390/ijms23094642. Int J Mol Sci. 2022. PMID: 35563035 Free PMC article. Review. - STED Imaging of Vesicular Endocytosis in the Synapse.
Hu S, Xie Z, Wang B, Chen Y, Jing Z, Hao Y, Yao J, Wu X, Huo J, Wei A, Qin Y, Dong N, Zheng C, Song Q, Long J, Kang X, Wang C, Xu H. Hu S, et al. Neurosci Bull. 2024 Sep;40(9):1379-1395. doi: 10.1007/s12264-024-01254-7. Epub 2024 Jul 8. Neurosci Bull. 2024. PMID: 38976218 - Tools for analysis and conditional deletion of subsets of sensory neurons.
Santana-Varela S, Bogdanov YD, Gossage SJ, Okorokov AL, Li S, de Clauser L, Alves-Simoes M, Sexton JE, Iseppon F, Luiz AP, Zhao J, Wood JN, Cox JJ. Santana-Varela S, et al. Wellcome Open Res. 2021 Sep 30;6:250. doi: 10.12688/wellcomeopenres.17090.1. eCollection 2021. Wellcome Open Res. 2021. PMID: 35233469 Free PMC article. - Thermal hyperalgesia and dynamic weight bearing share similar recovery dynamics in a sciatic nerve entrapment injury model.
Sheehan GD, Martin MK, Young VA, Powell R, Bhattacharjee A. Sheehan GD, et al. Neurobiol Pain. 2021 Dec 6;10:100079. doi: 10.1016/j.ynpai.2021.100079. eCollection 2021 Aug-Dec. Neurobiol Pain. 2021. PMID: 34917858 Free PMC article.
References
- Kawasaki Y, Zhang L, Cheng J-K, Ji R-R. Cytokine mechanisms of central sensitization: distinct and overlapping role of Interleukin-1β, Interleukin-6, and Tumor Necrosis Factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials