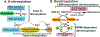The manifold roles of protein S-nitrosylation in the life of insulin - PubMed (original) (raw)
Review
The manifold roles of protein S-nitrosylation in the life of insulin
Hua-Lin Zhou et al. Nat Rev Endocrinol. 2022 Feb.
Abstract
Insulin, which is released by pancreatic islet β-cells in response to elevated levels of glucose in the blood, is a critical regulator of metabolism. Insulin triggers the uptake of glucose and fatty acids into the liver, adipose tissue and muscle, and promotes the storage of these nutrients in the form of glycogen and lipids. Dysregulation of insulin synthesis, secretion, transport, degradation or signal transduction all cause failure to take up and store nutrients, resulting in type 1 diabetes mellitus, type 2 diabetes mellitus and metabolic dysfunction. In this Review, we make the case that insulin signalling is intimately coupled to protein S-nitrosylation, in which nitric oxide groups are conjugated to cysteine thiols to form S-nitrosothiols, within effectors of insulin action. We discuss the role of S-nitrosylation in the life cycle of insulin, from its synthesis and secretion in pancreatic β-cells, to its signalling and degradation in target tissues. Finally, we consider how aberrant S-nitrosylation contributes to metabolic diseases, including the roles of human genetic mutations and cellular events that alter S-nitrosylation of insulin-regulating proteins. Given the growing influence of S-nitrosylation in cellular metabolism, the field of metabolic signalling could benefit from renewed focus on S-nitrosylation in type 2 diabetes mellitus and insulin-related disorders.
© 2021. Springer Nature Limited.
Conflict of interest statement
Competing interests
We declare that the authors have no competing financial or non-financial interests as defined by Nature journals’ policy.
Figures
Figure 1.. Working model of protein S-nitrosylation and denitrosylation.
(A) The complete machinery for enzymatic S-nitrosylation includes three classes of enzymes: NO synthases, SNO synthases and transnitrosylases. NO synthases catalyze the production of NO from L-arginine and include three members in mammals: eNOS, iNOS and nNOS. SNO synthases convert NO to NO+ through one-electron oxidation, and conjugate it to thiol to generate protein S-nitrosothiol. There are two identified SNO synthases: Hybrid cluster protein (HCP) in bacteria and hemoglobin in mammals (others are anticipated). Transnitrosylases propagate SNO-based signaling through transferring the SNO group from themselves to Cys residues on substrates. Multiple transnitrosylases have been identified. GAPDH and S100A9 are well-known examples. (B) There are two main classes of S-nitrosothiol denitrosylases: low molecular weight (LMW) thiol-co-factor dependent denitrosylases and thioredoxin (Trx)-related denitrosylases. Low molecular weight thiol (LMW)-SNOs, including SNO-glutathione (GSNO) and SNO-coenzyme A (SNO-CoA), are in equilibrium with SNO-proteins through transnitrosylation. GSNO reductase (GSNOR) and SNO-CoA reductase (SCoR) are enzymes that denitrosylate SNO-substrates by reducing GSNO and SNO-CoA, respectively. Trx/TrxR (thioredoxin reductase) systems denitrosylate SNO-proteins directly using vicinal thiols.
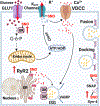
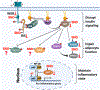
Figure 2.. Role of S-nitrosylation in insulin secretion from β-cells.
Mechanism of GSIS. Extracellular glucose enters β-cells and is phosphorylated by GCK, trapping phosphorylated glucose inside the cell and initiating its metabolism. The generation of ATP by glycolysis, the Krebs cycle and the respiratory chain in mitochondria lead to a rise in ATP, and a high ATP:ADP ratio closes ATP-sensitive KATP channels. The resulting membrane depolarization triggers the opening of voltage-dependent T-type calcium channels (VDCC) and influx of calcium ions (Ca2+). The release of intracellular ER Ca2+ is amplified by the rise of cytosolic calcium via RyR2 and IP3 receptors. Cytosolic Ca2+ induces the fusion of pre-docked insulin secretory granules (ISG) with the plasma membrane in the initial phase of insulin secretion. The rise of Ca2+ also induces new ISG to move to the plasma membrane for subsequent docking, fusion and insulin release. Interactions of VAMP on the ISG with Syn-4 and SNAP on the plasma membrane generates the active SNARE complex, mediating docking and fusion of ISG to release insulin. S-nitrosylation regulates insulin release through at least three steps: 1) S-nitrosylation of GCK increases cytosolic GCK activity by promoting the dissociation of GCK from ISG. Mutations in GCK that prevent S-nitrosylation thus cause diabetes. 2) S-nitrosylation of Syn-4 results in conformation changes of SNARE proteins and facilitates docking and fusion of ISG with the plasma membrane. 3) S-nitrosylation of RyR2 increases Ca2+ release. Hypo-nitrosylation of RyR2 causes Ca2+ leak, while hyper-nitrosylation leads to ER stress.
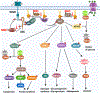
Figure 3.. Facilatory role of S-nitrosylation in the AKT branch of insulin signal transduction.
Binding of insulin to its receptor (INSR) triggers kinase activity that autophosphorylates tyrosine residues in INSR-β(pY) and phosphorylates tyrosine residues in IRS proteins. The SH2 domain of the p85 regulatory subunit of PI3K (bound to the p110 catalytic subunit of PI3K) binds to tyrosine-phosphorylated IRS to activate PI3K activity. PI3K catalyzes formation of phosphatidylinositol (3, 4, 5)-trisphosphate (PIP3) through adding a phosphate group to phosphatidylinositol (4, 5)-bisphosphate (PIP2). PIP3 binds to both PDK1 and AKT to activate these kinases. PDK1 phosphorylates AKT, thereby fully activating AKT. Activated AKT phosphorylates and inactivates TSC2, GSK3, FOXO, PDE3B and AS160 to control multiple aspects of insulin action, including the uptake of glucose, glycogen synthesis, lipogenesis, protein translation and cell proliferation. Protein S-nitrosylation elevates insulin responsiveness via modifying three phosphatases: 1) S-nitrosylation of PTP1B inhibits its phosphatase activity, prolonging activating-phosphorylation of both insulin receptor and IRS. 2) S-nitrosylation of PTEN inhibits dephosphorylation of the 3’ phosphate of the inositol ring in PIP3, leading to sustained PIP3 levels and AKT activity. 3) S-nitrosylation of SHIP2 inhibits dephosphorylation of the 5’ phosphate of the inositol ring in PIP3, also leading to sustained PIP3 levels.
Figure 4.. Mechanisms of inflammation-mediated insulin resistance.
Four mechanisms contribute to inflammatory signaling leading to insulin resistance. 1) TNF-α and IL-1β (from M1-like macrophages), FFAs (derived from adipocyte lipolysis), and LTB4 (from leukocytes) bind to receptors to activate downstream kinases such as c-Jun N-terminal kinase (JNK), IκB kinase β (IKK), protein kinase C (PKC), and ERK (MAPK). These activated kinases impair insulin signal transduction via insulin-independent serine/threonine phosphorylation of INSR and IRS, which reduces insulin-induced tyrosine phosphorylation of IRS. 2) IFN-γ from Th1 cells and IL-6 from M1-like macrophages activate JAK/STAT1/3 pathways that impair insulin signaling by increasing the expression of suppressor of cytokine signaling protein (SOCS), which interrupts the interaction of INSR with IRS and promotes their degradation. 3) JNK, IKKβ and JAK kinases, activated by either inflammatory mediators or sustained ER stress, trigger translocation of the transcriptional factors AP-1, NF-κB and STAT1/3 to the nucleus, where they initiate inflammatory gene expression to maintain the chronic inflammatory state that contributes to insulin resistance. 4) The transcription factors XBP1, ATF4 and ATF6 activated by the unfolded protein response (UPR) during FFA-induced ER stress initiate inflammatory gene expression, increase the inflammatory response and lead to insulin resistance.
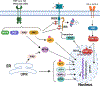
Figure 5.. Mechanisms of insulin resistance mediated by aberrant S-nitrosylation of proteins in insulin signaling cascades.
Three mechanisms are known for how insulin signal transduction is regulated by protein S-nitrosylation to promote insulin resistance. 1) S-nitrosylation of INSR-β, IRS1 and AKT1 inhibit their activity or promote their degradation, disrupting insulin signaling. 2) S-nitrosylation of PPARγ and PDE3B inhibit their activity and impair adipocyte function, thereby leading to insulin resistance of adipocytes. 3) S-nitrosylation of SIRT1 blocks its deacetylase activity and augments acetylation of NF-kB p65, thereby activating NF-κB transcriptional activity. Activated NF-κB initiates the expression of pro-inflammatory genes to maintain the inflammatory state that reinforces insulin resistance. NF-κB and IKK can also be S-nitrosylated, but whether this plays a role in IR is not known.
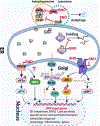
Figure 6.. The role of aberrant protein S-nitrosylation in ER stress-mediated insulin resistance.
The UPR pathway is mediated by three transmembrane proteins: IRE1, PERK, and ATF6. Upon ER stress, 1) PERK dimerizes to activate its kinase, and phosphorylates eIF2α to decrease global protein translation, but selectively activates the translation of ATF4. PERK also phosphorylates NRF2. ATF4 and NRF2 initiate the transcription of genes involved in the antioxidant response and amino acid transport, thereby alleviating ER stress; 2) ATF-6α traffics from the ER to the Golgi, where it is proteolytically activated to release the cytosolic B-ZIP transcription factor domain of ATF-6α that translocates to the nucleus, where it acts as a transcriptional factor to upregulate UPR target gene expression to protect the cell from ER stress; 3) IRE1 dimerization and autophosphorylation activate its endoribonuclease activity, which is responsible for removing 26 nucleotides from the X box-binding protein 1 (XBP1) mRNA via an unconventional splicing event. Spliced Xbp-1 encodes a potent transcription factor of the basic-leucine zipper (B-ZIP) family, which induces ERAD proteins and chaperones. The ER protein quality control system includes protein re-folding apparatus (ER-resident folding chaperones), ERAD and autophagy. There are at least three mechanisms for how protein S-nitrosylation affects the UPR pathway and protein quality control systems in ER stress, eventually leading to IR. 1) S-nitrosylation of IRE1 prevents the generation of sXBP1 by inhibiting IRE1 endoribonuclease activity. 2) S-nitrosylation of the chaperone PDI inhibits its enzymatic activity, leading to accumulation of misfolded proteins. 3) S-nitrosylation of the lysosomal proteins HexB and CTSB inhibit these autophagy-related lysosomal enzymes, blocking autophagy. Although PEAK, BiP and UBE2D1 also can be S-nitrosylated in certain conditions or cell types, the link to IR remains unclear.
Similar articles
- Nutrient regulation of insulin secretion and action.
Newsholme P, Cruzat V, Arfuso F, Keane K. Newsholme P, et al. J Endocrinol. 2014 Jun;221(3):R105-20. doi: 10.1530/JOE-13-0616. Epub 2014 Mar 25. J Endocrinol. 2014. PMID: 24667247 Review. - Stimulus-induced S-nitrosylation of Syntaxin 4 impacts insulin granule exocytosis.
Wiseman DA, Kalwat MA, Thurmond DC. Wiseman DA, et al. J Biol Chem. 2011 May 6;286(18):16344-54. doi: 10.1074/jbc.M110.214031. Epub 2011 Mar 10. J Biol Chem. 2011. PMID: 21393240 Free PMC article. - Naturally occurring glucokinase mutations are associated with defects in posttranslational S-nitrosylation.
Ding SY, Tribble ND, Kraft CA, Markwardt M, Gloyn AL, Rizzo MA. Ding SY, et al. Mol Endocrinol. 2010 Jan;24(1):171-7. doi: 10.1210/me.2009-0138. Epub 2009 Nov 24. Mol Endocrinol. 2010. PMID: 19934346 Free PMC article. - Metabolic signaling of insulin secretion by pancreatic β-cell and its derangement in type 2 diabetes.
Zou CY, Gong Y, Liang J. Zou CY, et al. Eur Rev Med Pharmacol Sci. 2014;18(15):2215-27. Eur Rev Med Pharmacol Sci. 2014. PMID: 25070829 Retracted. Review.
Cited by
- Impact of Reactive Species on Amino Acids-Biological Relevance in Proteins and Induced Pathologies.
Andrés CMC, Pérez de la Lastra JM, Andrés Juan C, Plou FJ, Pérez-Lebeña E. Andrés CMC, et al. Int J Mol Sci. 2022 Nov 14;23(22):14049. doi: 10.3390/ijms232214049. Int J Mol Sci. 2022. PMID: 36430532 Free PMC article. Review. - The enzymatic function of the honorary enzyme: S-nitrosylation of hemoglobin in physiology and medicine.
Premont RT, Singel DJ, Stamler JS. Premont RT, et al. Mol Aspects Med. 2022 Apr;84:101056. doi: 10.1016/j.mam.2021.101056. Epub 2021 Nov 28. Mol Aspects Med. 2022. PMID: 34852941 Free PMC article. Review. - Metabolic regulation of glucagon secretion.
Armour SL, Stanley JE, Cantley J, Dean ED, Knudsen JG. Armour SL, et al. J Endocrinol. 2023 Sep 8;259(1):e230081. doi: 10.1530/JOE-23-0081. Print 2023 Sep 1. J Endocrinol. 2023. PMID: 37523232 Free PMC article. - Inhibition of NOS1 promotes the interferon response of melanoma cells.
Chen X, Zou Z, Wang Q, Gao W, Zeng S, Ye S, Xu P, Huang M, Li K, Chen J, Zhong Z, Zhang Q, Hao B, Liu Q. Chen X, et al. J Transl Med. 2022 May 10;20(1):205. doi: 10.1186/s12967-022-03403-w. J Transl Med. 2022. PMID: 35538490 Free PMC article. - An enzyme that selectively S-nitrosylates proteins to regulate insulin signaling.
Zhou HL, Grimmett ZW, Venetos NM, Stomberski CT, Qian Z, McLaughlin PJ, Bansal PK, Zhang R, Reynolds JD, Premont RT, Stamler JS. Zhou HL, et al. Cell. 2023 Dec 21;186(26):5812-5825.e21. doi: 10.1016/j.cell.2023.11.009. Epub 2023 Dec 5. Cell. 2023. PMID: 38056462 Free PMC article.
References
- Sonksen P and Sonksen J (2000) Insulin: understanding its action in health and disease. Br J Anaesth 85 (1), 69–79. - PubMed
- Weiss M et al. (2000) Insulin Biosynthesis, Secretion, Structure, and Structure-Activity Relationships. In Endotext (Feingold KR et al. eds). - PubMed
- Kurohane Kaneko Y and Ishikawa T (2013) Dual role of nitric oxide in pancreatic beta-cells. J Pharmacol Sci 123 (4), 295–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL126900/HL/NHLBI NIH HHS/United States
- R01 HL157151/HL/NHLBI NIH HHS/United States
- P01 HL158507/HL/NHLBI NIH HHS/United States
- R01 DK128347/DK/NIDDK NIH HHS/United States
- R01 DK119506/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical