SARS-CoV-2 spike S1 subunit induces neuroinflammatory, microglial and behavioral sickness responses: Evidence of PAMP-like properties - PubMed (original) (raw)
SARS-CoV-2 spike S1 subunit induces neuroinflammatory, microglial and behavioral sickness responses: Evidence of PAMP-like properties
Matthew G Frank et al. Brain Behav Immun. 2022 Feb.
Abstract
SARS-CoV-2 infection produces neuroinflammation as well as neurological, cognitive (i.e., brain fog), and neuropsychiatric symptoms (e.g., depression, anxiety), which can persist for an extended period (6 months) after resolution of the infection. The neuroimmune mechanism(s) that produces SARS-CoV-2-induced neuroinflammation has not been characterized. Proposed mechanisms include peripheral cytokine signaling to the brain and/or direct viral infection of the CNS. Here, we explore the novel hypothesis that a structural protein (S1) derived from SARS-CoV-2 functions as a pathogen-associated molecular pattern (PAMP) to induce neuroinflammatory processes independent of viral infection. Prior evidence suggests that the S1 subunit of the SARS-CoV-2 spike protein is inflammatory in vitro and signals through the pattern recognition receptor TLR4. Therefore, we examined whether the S1 subunit is sufficient to drive 1) a behavioral sickness response, 2) a neuroinflammatory response, 3) direct activation of microglia in vitro, and 4) activation of transgenic human TLR2 and TLR4 HEK293 cells. Adult male Sprague-Dawley rats were injected intra-cisterna magna (ICM) with vehicle or S1. In-cage behavioral monitoring (8 h post-ICM) demonstrated that S1 reduced several behaviors, including total activity, self-grooming, and wall-rearing. S1 also increased social avoidance in the juvenile social exploration test (24 h post-ICM). S1 increased and/or modulated neuroimmune gene expression (Iba1, Cd11b, MhcIIα, Cd200r1, Gfap, Tlr2, Tlr4, Nlrp3, Il1b, Hmgb1) and protein levels (IFNγ, IL-1β, TNF, CXCL1, IL-2, IL-10), which varied across brain regions (hypothalamus, hippocampus, and frontal cortex) and time (24 h and 7d) post-S1 treatment. Direct exposure of microglia to S1 resulted in increased gene expression (Il1b, Il6, Tnf, Nlrp3) and protein levels (IL-1β, IL-6, TNF, CXCL1, IL-10). S1 also activated TLR2 and TLR4 receptor signaling in HEK293 transgenic cells. Taken together, these findings suggest that structural proteins derived from SARS-CoV-2 might function independently as PAMPs to induce neuroinflammatory processes via pattern recognition receptor engagement.
Keywords: Microglia; Neuroinflammation; PAMP; S1 subunit; SARS-CoV-2; Sickness behavior; Spike protein; TLR.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Fig. 1
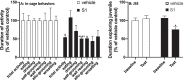
Effect of S1 on behavior. Rats were injected ICM with vehicle (1x PBS) or S1 (1 μg). (A) 8 h after ICM injection, home cage infrared video recordings were made of in-cage behaviors from 1 h pre- to 1 h post-lights off and duration of behaviors scored based on Stanford Ethogram definitions. (B) JSE was scored 24 h prior to ICM injection (baseline) and 24 h after ICM injection (test). Data are presented as the mean + SEM. N = 6/group. S1 vs vehicle, *p < 0.05, ****p < 0.0001.
Fig. 2
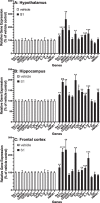
Effects of S1 24 h post-ICM injection on neuroinflammatory genes. Rats were injected ICM with vehicle (1x PBS) or S1 (1 μg). 24 h after ICM injection, gene expression of glial activation markers and neuroinflammatory-related genes was measured in (A) hypothalamus, (B) hippocampus and (C) frontal cortex. Data are presented as the mean + SEM. N = 6/group. S1 vs vehicle, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Fig. 3
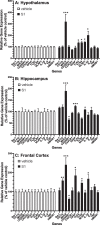
Effects of S1 7d post-ICM injection on neuroinflammatory genes. Rats were injected ICM with vehicle (1x PBS) or S1 (1 μg). 7d after ICM injection, gene expression of glial activation markers and neuroinflammatory-related genes was measured in (A) hypothalamus, (B) hippocampus and (C) frontal cortex. Data are presented as the mean + SEM. N = 6/group. S1 vs vehicle, *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 4
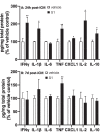
Effects of S1 24 h and 7d post-ICM injection on neuroinflammatory proteins. Rats were injected ICM with vehicle (1x PBS) or S1 (1 μg). Protein levels of neuroinflammatory-related proteins were measured in hippocampus (A) 24 h and (B) 7d after ICM injection. Data are presented as the mean + SEM. N = 5–6/group. S1 vs vehicle, *p < 0.05, **p < 0.01.
Fig. 5
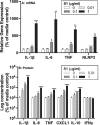
Proinflammatory effects of S1 in isolated microglia. Whole brain microglia were isolated from adult rats and exposed to several concentrations of S1 (0, 0.01, 0.1, and 1 μg/ml) for 24 h. (A) RNA was isolated from cells and proinflammatory gene expression measured and (B) protein levels were measured in cell culture supernatants. Data are presented as the mean + SEM. N = 3 replications. S1 concentration vs media control, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Fig. 6
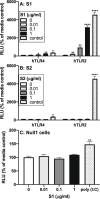
Effect of S1 and S2 on hTLR2 and hTLR4 signaling. HEK293 cells expressing hTLR2 or hTLR4 were exposed to several concentrations of (A) S1 (0, 0.01, 0.1, and 1 μg/ml) or (B) S2 (0, 0.01, 0.1, and 1 μg/ml) for 4 h and SEAP expression measured in supernatants. (C) Null1 cells were exposed to S1 (0, 0.01, 0.1, and 1 μg/ml) for 4 h and SEAP expression measured in supernatants. hTLR2 control = 100 ng/ml PAM3csk4; hTLR4 control = 100 ng/ml LPS; Null1 control (TLR3) = 100 ng/ml poly I:C. Data are presented as the mean + SEM. N = 3 replications. S1 concentration vs media control, *p < 0.05, **p < 0.01, ****p < 0.0001.
Similar articles
- SARS-CoV-2 S1 subunit produces a protracted priming of the neuroinflammatory, physiological, and behavioral responses to a remote immune challenge: A role for corticosteroids.
Frank MG, Ball JB, Hopkins S, Kelley T, Kuzma AJ, Thompson RS, Fleshner M, Maier SF. Frank MG, et al. Brain Behav Immun. 2024 Oct;121:87-103. doi: 10.1016/j.bbi.2024.07.034. Epub 2024 Jul 21. Brain Behav Immun. 2024. PMID: 39043345 - The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: A role for the NLRP3 inflammasome.
Frank MG, Weber MD, Fonken LK, Hershman SA, Watkins LR, Maier SF. Frank MG, et al. Brain Behav Immun. 2016 Jul;55:215-224. doi: 10.1016/j.bbi.2015.10.009. Epub 2015 Oct 19. Brain Behav Immun. 2016. PMID: 26482581 Free PMC article. - SARS-CoV-2 Spike Glycoprotein S1 Induces Neuroinflammation in BV-2 Microglia.
Olajide OA, Iwuanyanwu VU, Adegbola OD, Al-Hindawi AA. Olajide OA, et al. Mol Neurobiol. 2022 Jan;59(1):445-458. doi: 10.1007/s12035-021-02593-6. Epub 2021 Oct 28. Mol Neurobiol. 2022. PMID: 34709564 Free PMC article. - Exploring the immunogenic properties of SARS-CoV-2 structural proteins: PAMP:TLR signaling in the mediation of the neuroinflammatory and neurologic sequelae of COVID-19.
Frank MG, Fleshner M, Maier SF. Frank MG, et al. Brain Behav Immun. 2023 Jul;111:259-269. doi: 10.1016/j.bbi.2023.04.009. Epub 2023 Apr 27. Brain Behav Immun. 2023. PMID: 37116592 Free PMC article. Review. - Role of SARS-CoV-2 Spike-Protein-Induced Activation of Microglia and Mast Cells in the Pathogenesis of Neuro-COVID.
Theoharides TC, Kempuraj D. Theoharides TC, et al. Cells. 2023 Feb 22;12(5):688. doi: 10.3390/cells12050688. Cells. 2023. PMID: 36899824 Free PMC article. Review.
Cited by
- Long COVID endotheliopathy: hypothesized mechanisms and potential therapeutic approaches.
Ahamed J, Laurence J. Ahamed J, et al. J Clin Invest. 2022 Aug 1;132(15):e161167. doi: 10.1172/JCI161167. J Clin Invest. 2022. PMID: 35912863 Free PMC article. Review. - Microglia Involves in the Immune Inflammatory Response of Poststroke Depression: A Review of Evidence.
Xia W, Xu Y, Gong Y, Cheng X, Yu T, Yu G. Xia W, et al. Oxid Med Cell Longev. 2022 Aug 2;2022:2049371. doi: 10.1155/2022/2049371. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35958023 Free PMC article. Retracted. Review. - Long-term effects of SARS-CoV-2 infection on human brain and memory.
Ding Q, Zhao H. Ding Q, et al. Cell Death Discov. 2023 Jun 29;9(1):196. doi: 10.1038/s41420-023-01512-z. Cell Death Discov. 2023. PMID: 37380640 Free PMC article. Review. - SARS-CoV-2 envelope protein triggers depression-like behaviors and dysosmia via TLR2-mediated neuroinflammation in mice.
Su W, Ju J, Gu M, Wang X, Liu S, Yu J, Mu D. Su W, et al. J Neuroinflammation. 2023 May 8;20(1):110. doi: 10.1186/s12974-023-02786-x. J Neuroinflammation. 2023. PMID: 37158916 Free PMC article. - Microglial Inflammatory Responses to SARS-CoV-2 Infection: A Comprehensive Review.
Dey R, Bishayi B. Dey R, et al. Cell Mol Neurobiol. 2023 Dec 15;44(1):2. doi: 10.1007/s10571-023-01444-3. Cell Mol Neurobiol. 2023. PMID: 38099973 Review.
References
- Benameur K., Agarwal A., Auld S.C., Butters M.P., Webster A.S., Ozturk T., Howell J.C., Bassit L.C., Velasquez A., Schinazi R.F., Mullins M.E., Hu W.T. Encephalopathy and Encephalitis Associated with Cerebrospinal Fluid Cytokine Alterations and Coronavirus Disease, Atlanta, Georgia, USA, 2020. Emerg. Infect. Dis. 2020;26(9):2016–2021. - PMC - PubMed
- Bianchi M.E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81(1):1–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous