Mechanistic Role of Jak3 in Obesity-Associated Cognitive Impairments - PubMed (original) (raw)
Mechanistic Role of Jak3 in Obesity-Associated Cognitive Impairments
Premranjan Kumar et al. Nutrients. 2022.
Abstract
Background and aims: A compromise in intestinal mucosal functions is associated with several chronic inflammatory diseases. Previously, we reported that obese humans have a reduced expression of intestinal Janus kinase-3 (Jak3), a non-receptor tyrosine kinase, and a deficiency of Jak3 in mice led to predisposition to obesity-associated metabolic syndrome. Since meta-analyses show cognitive impairment as co-morbidity of obesity, the present study demonstrates the mechanistic role of Jak3 in obesity associated cognitive impairment. Our data show that high-fat diet (HFD) suppresses Jak3 expression both in intestinal mucosa and in the brain of wild-type mice.
Methodology: Recapitulating these conditions using global (Jak3-KO) and intestinal epithelial cell-specific conditional (IEC-Jak3-KO) mice and using cognitive testing, western analysis, flow cytometry, immunofluorescence microscopy and 16s rRNA sequencing, we demonstrate that HFD-induced Jak3 deficiency is responsible for cognitive impairments in mice, and these are, in part, specifically due to intestinal epithelial deficiency of Jak3.
Results: We reveal that Jak3 deficiency leads to gut dysbiosis, compromised TREM-2-functions-mediated activation of microglial cells, increased TLR-4 expression and HIF1-α-mediated inflammation in the brain. Together, these lead to compromised microglial-functions-mediated increased deposition of β-amyloid (Aβ) and hyperphosphorylated Tau (pTau), which are responsible for cognitive impairments. Collectively, these data illustrate how the drivers of obesity promote cognitive impairment and demonstrate the underlying mechanism where HFD-mediated impact on IEC-Jak3 deficiency is responsible for Jak3 deficiency in the brain, reduced microglial TREM2 expression, microglial activation and compromised clearance of Aβ and pTau as the mechanism during obesity-associated cognitive impairments.
Conclusion: Thus, we not only demonstrate the mechanism of obesity-associated cognitive impairments but also characterize the tissue-specific role of Jak3 in such conditions through mucosal tolerance, gut-brain axis and regulation of microglial functions.
Keywords: Janus kinase-3; TREM-2; cognitive impairments; high-fat diet; microglia; obesity.
Conflict of interest statement
The authors declare that they have no conflict of interest in terms of the contents of this article.
Figures
Figure 1
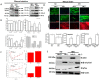
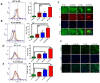
High-fat diet (HFD) reduces intestinal expression of Jak3, and Jak3 deficiency in mice leads to colonic dysbiosis, cognitive impairment and accumulation of β-Amyloid and pTau in obese mouse brain. (A) Colons from normal diet (ND) and HFD-fed mice were excised out, and Western blot analysis was performed using the tissue lysates for the indicated proteins using β-actin as controls. Representative blots (n = 3) are shown. (B) Jak3 deficiency promotes gut dysbiosis. Gut microbiome composition was determined using a commercial facility through 16sRNA pyrosequencing of fecal DNA samples from co-housed WT and Jak3-KO littermates (n = 10 each group). The time courses of changes in fecal microbiota from WT and Jak3-KO mice are shown through the shift in the relative abundance of specific taxa (Upper panels; UP), specific family (Lower left panel; LLP), and the time courses of the relative shift in Firmicutes to Bacteroidetes ratio (F/B) (Lower right panel; LRP) are shown. Statistical analysis was performed using repeated measures paired group analysis of variance. Error bars represent +/−SEM. * indicate statistically significant differences compared to WT (ULP; p = 0.004, URP; p = 0.002, LLP; p = 0.001). (C) Jak3 deficiency promotes cognitive impairment. Automated elevated radial arm maze equipped with MazesoftTM Software was used to calculate the four parameters of cognitive assessments, viz., working memory errors (WME) (Upper left), roaming memory errors (RME) (Upper right), number of errors before a correct choice (EBCC) (Lower left) and reward memory errors (RWME) (Lower Right) over five sessions. A repeated-measure ANOVA on performances of age- and sex-matched wild-type (WT) littermate controls of Jak3-KO mice (n = 20 each group) are shown for each session (Upper and lower left panels) or an average of all the sessions (Upper and lower right panels). All the data are representative of three independent experiments. Values are mean ± S.D. * denotes p < 0.05 compared with WT mice. (Upper left; p = 0.002, Lower left; p = 0.004, Lower right; p = 0.001). (D) Jak3 deficiency promotes accumulation of β-Amyloid and pTau in mouse brain. Brain tissue sections from WT and corresponding Jak3-KO littermate mice fed with either ND or HFD were immunostained using β-amyloid or pTau or Jak3 primary antibodies followed by FITC- or Cy-3-conjugated secondary antibodies. Mounting media containing PI were used to visualize the nucleus. Representative images (n = 10) are shown (Lower panels). Quantifications of the intensities in the “upper panels” were performed using NikonR C1-plus imaging software, and the results were normalized against PI for Jak3 (Lower left), β-amyloid (Lower middle) and pTau (Lower right). Values are mean ± S.D. Asterisk denotes p < 0.05 compared with WT mice group. (Jak3 p = 0.003, β-amyloid p = 0.005, pTau p = 0.002). (E) Brain tissue lysates from the samples in “D” were used to perform IB in the presence of the indicated antibodies with β-actin as control. Representative blots (n = 3) are shown.
Figure 2
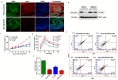
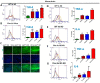
Intestinal epithelial cell (IEC) deficiency of Jak3 predisposes to exaggerated symptoms of HFD-induced obesity and glycemic dysregulation. (A), Genetic manipulation shows IEC deficiency of Jak3. Colonic tissue sections from floxed Jak3 (jak3f/f) or IEC-Jak3-KO (vil1-Cre- jak3f/f) were co-immunostained with IEC marker villin (green) and Jak3 (red) antibodies using DAPI as control for the nucleus. Representative images (n = 10) are shown from each group (n = 6). (B), Western blot analyses were performed using tissue lysates from floxed Jak3 and IEC-Jak3-KO mice for Jak3 expression (upper panel) using β-actin as control (lower panel). Representative blots (n = 3) are shown. (C), Fluorescence-activated cell sorting (FACS) analysis is presented as four quadrant dot plots to determine the impact of HFD on IEC-Jak3 expression by determining the double positive cells (Quadrant:UR) for IEC marker villin and Jak3 under ND or HFD. Jak3 positive cells are shown on the x axis and those of villin positive cells on the y axis. (D), Bar chart results following FACS experiments in “C” were repeated (n = 5), and mean ± SD values are shown as bar graph for the comparative average cell counts for the indicated groups of mice. *** denotes p = 0.004 (Int-KO ND), p = 0.008 (FL HFD), p = 0.001(Int-KO HFD). (E), IEC deficiency of Jak3 predisposes to increased body weight. Vil1-Cre/ jak3f/f (Int-KO) or their littermate controls jak3f/f (FL) mice were cohoused and subjected to either ND or HFD. Body weights of co-housed Int-KO and their FL littermate males were plotted at the indicated intervals as a percentage of their body weight at the time of weaning (n = 5/group). *** denotes p = 0.005 (Int-KO HFD), p = 0.05 (Int-KO ND). (F), IEC deficiency of Jak3 impairs the ability to restore blood glucose. Blood glucose concentrations in fasted mice were measured after intraperitoneal injection with 2 g/kg body weight of d-glucose followed by measuring the level of blood glucose at thew, indicating intervals post-injection (n = 5/group). *** denotes p = 0.003 (Int-KO HFD), p = 0.05 (Int-KO ND).
Figure 3
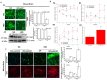
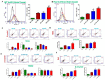
IEC deficiency in Jak3 is responsible for cognitive impairment and increased cerebral cortex accumulation of Aβ and pTau during HFD-induced obesity: (A) IEC-Jak3 deficiency promotes HFD-mediated impacts on Jak3 expression in the brain in mice. Brain tissue sections (A1) or tissue-lysates (A2) from FL-control and corresponding IEC-Jak3-KO littermate mice fed with either ND or HFD were immunostained or Western blotted using Jak3 primary antibodies followed by FITC- or HRP-conjugated secondary antibodies, respectively. Mounting media containing PI were used to visualize the nucleus in A1. Representative images in A1 or blots in A2 (n = 10 and 3, respectively) are shown (Right panels). Quantifications of the florescent intensities in A1 or densitometric analyses in A2 in the corresponding “left panels” were performed using NikonR C1-plus imaging and BioRad software, respectively, and the results were normalized against PI in B1 or β-actin in B2 for Jak3 expression. (B) IEC-Jak3 deficiency promotes cognitive impairment during obesity. The four parameters of cognitive assessments, viz., WME (B1), EBCC (B2), RME (B3) and RWME (B4), were measured, as in Figure 1C. A repeated-measure ANOVA on performances of age- and sex-matched Jak3-flox (Flox-control) and littermate controls of IEC-Jak3-KO mice (n = 10 each group) is shown for each session (B1–3) or an average of all the sessions (B4). Data are representative of three independent experiments. Values are mean ± S.D. * denotes statistically significant compared with flox mice. (A1; p > 0.01, A2; p > 0.05, A3; p > 0.01, A4). (C) IEC-Jak3 deficiency promotes accumulation of β-Amyloid and pTau in mouse brain. Brain tissue sections from FL-control and corresponding IEC-Jak3-KO littermate mice fed with either ND or HFD were immunostained using β-amyloid or pTau or Jak3 primary antibodies followed by FITC- or Cy-3-conjugated secondary antibodies. Mounting media containing PI were used to visualize the nucleus. Representative images (n = 10) are shown (Right panels). Quantifications of the intensities in the “left panels” were performed using NikonR C1-plus imaging software, and the results were normalized against PI for β-amyloid (Upper right) and pTau (Lower right). Values are mean ± S.D. Asterisk denotes statistically significant p > 0.05 compared with flox mice group. (β-amyloid p = 0.005, pTau p = 0.003).
Figure 4
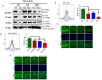
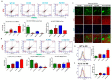
The triggering receptors on microglial cells 2 (TREM2) interact with Jak3 in the brain, and the intestinal deficiency of Jak3 leads to reduced interactions and expression of TREM-2 in the brain with Jak3 during HFD-induced obesity: (A) Jak3 interactions with TREM-2 were determined using brain tissue lysates from ND- and HFD-fed mice. Lysates from WT (littermate control), Jak3-KO, IEC-Jak3-KO and flox-Jak3 (littermate control) were subjected to IP followed by IB using the indicated antibodies. Blots shown represent n = 3 experiments. (B) The impact of global and intestinal epithelial tissue-specific deficiencies of Jak3 on the brain expression of β-Amyloid receptor TREM2 on microglial cells was determined in ND- and HFD-fed mouse brain. Representative flow cytometric histogram graphs of individual mouse brain cells showing the microglial levels of expression of TREM-2 receptor in the four groups (n = 5/group; WT-ND (littermate control), Jak3-KO-ND, WT-HFD (littermate control), Jak3-KO-HFD) are shown in the left panel, and the corresponding histogram bar graphs indicating mean ± SD values are shown for the comparative average cell counts for the indicated groups of mice. * Indicate statistically significant difference from the corresponding controls (KO-ND p = 0.05, KO-HFD p = 0.03). ** Comparison between Int-KO ND and Int-KO HFD group. (C) Brain tissue sections from WT-control littermate and Jak3-KO mice fed with either ND or HFD were immunostained using TREM-2 primary antibodies followed by FITC secondary antibodies, and mounting media containing PI were used to visualize the nucleus. Representative images (n = 10) are shown. (D) Data from similar experiments as in “B” but for the four different groups of mice (n = 5/group; flox-Jak3-ND (littermate control), IEC-Jak3-KO-ND, flox-Jak3-HFD (littermate control), IEC-Jak3-KO-HFD) are shown. * Indicate statistically significant difference from the corresponding controls (IEC-Jak3-KO-ND p = 0.05, KO-HFD p = 0.05). (E) Brain tissue sections from flox-Jak3-control littermate and IEC-Jak3-KO mice fed with either ND or HFD were immunostained using TREM-2 primary antibodies followed by FITC secondary antibodies, and mounting media containing PI were used to visualize the nucleus. Representative images (n = 10) are shown.
Figure 5
Intestinal deficiency of Jak3 leads to increased microglial activation in the brain during HFD-induced obesity. The impact of global (A,D) and intestinal epithelial tissue-specific (B,E) deficiencies of Jak3 on brain expression of microglial marker TREM-2 was determined in ND- and HFD-fed mouse brain. Representative flow cytometric histogram graphs of individual mouse brain cells showing the microglial levels of expression of Iba1 in the four indicated groups of global deficiency or IEC deficiency, respectively (n = 5/group), are shown in the left panel, and the corresponding histogram bar graphs indicating mean ± SD values in the right panel are shown for the comparative average cell counts for the indicated groups of mice. *, **, *** Indicate statistically significant difference from the corresponding controls ((A): KO-HFD#1 p = 0.05, KO-HFD#2 p = 0.03; (B): IEC-KO-HFD#1 p = 0.05, IEC-KO-HFD#2 p = 0.04; (D): WT-HFD _p_= 0.05, KO-HFD p = 0.03; (E): FL-HFD p = 0.07, IEC-KO-HFD p = 0.04). (C) Brain tissue sections from flox-Jak3-control littermate and IEC-Jak3-KO mice fed with either ND or HFD were immunostained using microglial marker Iba1 primary antibodies followed by Cy3 secondary antibodies, and mounting media containing PI were used to visualize the nucleus. Representative images (n = 10) are shown. (E) Brain tissue sections from flox-Jak3-control littermate and IEC-Jak3-KO mice fed with either ND or HFD were immunostained using microglial activation marker CD11b primary antibodies followed by FITC secondary antibodies, and mounting media containing PI were used to visualize the nucleus. (F) Similar experiments were performed as in “C” except the brain tissue sections were immunostained using microglial activation marker CD11b primary antibodies followed by FITC-conjugated secondary antibodies. (C&F) Representative images (n = 10) are shown.
Figure 6
HFD-led suppression of Jak3 promotes brain inflammation through microglial activation and increased TLR4 signaling. Global (A,D,E) or intestinal epithelial (B,F,G) deficiency of Jak3 leads to increased TLR4 expression and inflammation in the brain. Representative flow cytometric histogram graphs of individual mouse brain cells showing the levels of expression of TLR-4 (A,B) and inflammatory cytokines TNF-α (D,F) and IL-6 (E,G) in the four indicated groups of global Jak3 deficiency or IEC Jak3 deficiency, respectively (n = 5/group), are shown in the left panel, and the corresponding histogram bar graphs indicating mean ± SD values in the right or lower panels are shown for the comparative average cell counts for the indicated groups of mice. *, **, *** Indicate statistically significant difference from the corresponding controls (A: KO-ND#1 p = 0.04, KO-HFD#2 p = 0.07; B: IEC-KO-ND#1 p = 0.05, IEC-KO-HFD#2 p = 0.05; D: KO-ND p = 0.03, WT-HFD p = 0.01, KO-HFD p = 0.02; E: KO-ND p = 0.03, WT-HFD p = 0.01, KO-HFD p = 0.02; F: FL-HFD p = 0.06, IEC-KO-HFD p = 0.02)). (C) Brain tissue sections from flox-Jak3-control littermate and IEC-Jak3-KO mice fed with either ND or HFD were immunostained using TLR4 primary antibodies followed by FITC secondary antibodies, and mounting media containing PI were used to visualize the nucleus. Representative images (n = 10) are shown.
Figure 7
Intestinal epithelial deficiency of Jak3 causes Brain-hypoxia led increase in microglial accumulation of Abeta in the brain. (A,B), Representative flow cytometric histogram graphs of individual mouse brain cells showing the levels of expression of hypoxia inducible factor 1-α (HIF1-α) in the four indicated groups of global Jak3 deficiency (A) and IEC Jak3 deficiency (B), respectively (n = 5/group), are shown in the left panel, and the corresponding histogram bar graphs indicating mean ± SD values in the right panels are shown for the comparative average cell counts for the indicated groups of mice. *, **, *** Indicate statistically significant difference from the corresponding controls (A: KO-ND#1 p = 0.06, WT-HFD#2 p = 0.05 KO-HFD#3 p = 0.04; B: Int-KO-ND#1 p = 0.06, FL-HFD#2 p = 0.05, Int-KO-HFD#3 p = 0.05). (C,D), FACS analysis is presented as four quadrant dot plots to determine the impact of global Jak3 deficiency on microglial accumulation of Abeta in the individual mouse brain tissues from “A” by determining the double positive cells (Quadrant:UR) for microglial Abeta receptor TREM-2 on the X axis and Abeta on the Y axis (C) and microglial marker Iba1 on the X axis and Abeta on the Y axis (D) under ND or HFD. The lower panels in “C” and “D” show the corresponding bar chart results following repeating (n = 5) the FACS experiments, and mean ± SD values are shown as bar graph for the comparative average cell counts for the indicated groups of mice. *, **, *** denote p < 0.04 in all groups. (E,F), similar strategies as in C-D were used to perform FACS analysis to determine the impact of intestinal epithelial Jak3 deficiency on microglial accumulation of Abeta in the individual mouse brain tissues from “B” in the indicated four groups under ND or HFD. *, **, *** denote statistically significant values in the indicated group assumed at p < 0.05.
Figure 8
Intestinal epithelial cell deficiency of Jak3 causes increased microglial activation associated microglia accumulation of pTau in the brain. (A), FACS analysis is presented as four quadrant dot plots to determine the impact of global Jak3 deficiency on microglial accumulation of pTau in the individual mouse brain tissues by determining the double positive cells (Quadrant:UR) for microglial pTau receptor TREM-2 on the X axis and pTau on the Y axis in mice fed with either ND or HFD. The lower panels show the corresponding bar chart results following repeating (n = 5) the FACS experiments and mean ± SD values are shown as bar graph for the comparative average cell counts for the indicated groups of mice. *, ** denotes p < 0.04 in all groups. (B), similar strategy as in “A” was used, except using intestinal epithelial Jak3-deficient mouse brains to determine microglial accumulation of Abeta in the individual mouse brain tissues from the indicated four groups under ND or HFD. *, **, *** denotes statistically significant values in the indicated group assumed at p < 0.05. (C), Brain tissue sections from flox-Jak3-control littermate and IEC-Jak3-KO mice fed with either ND or HFD were immunostained using either microglial activation marker F4/80 or pTau primary antibodies followed by FITC and Cy3 secondary antibodies, respectively, and mounting media containing PI were used to visualize the nucleus. Representative images (n = 10) are shown. (D,E), Representative flow cytometric histogram graphs of individual mouse brain cells showing the levels of expression of F4/80 in the four indicated groups of global Jak3 deficiency (D) and IEC Jak3 deficiency (E), respectively (n = 5/group), are shown in the left panels, with the corresponding histogram bar graph indicating mean ± SD values in the right panels for the comparative average cell counts for the indicated groups of mice. **, *** denotes statistically significant values in the indicated group assumed at p < 0.05.
Figure 9
Intestinal epithelial cell deficiency of Jak3 causes suppressed microglial Iba1 associated increased accumulation of pTau in the brain. (A,B), FACS analysis is presented as four quadrant dot plots to determine the impact of either global Jak3 deficiency (A) or IEC Jak3 deficiency (B) on microglial accumulation of pTau in the individual mouse brain tissues by determining the double positive cells (Quadrant:UR) for microglial marker Iba1 on the X axis and pTau on the Y axis in mice fed with either ND or HFD. The lower panels show the corresponding bar chart results following repeating (n = 5) the FACS experiments, and mean ± SD values are shown as bar graph for the comparative average cell counts for the indicated groups of mice. *, **, *** denotes p < 0.04 in all groups. (C) Microglial internalization of pTau is compromised in IEC-Jak3-deficient mice. Brain tissue sections from flox-Jak3-control littermate and IEC-Jak3-KO mice fed with HFD were co-immunostained using microglial marker Iba1 and pTau primary antibodies followed by FITC and Cy3 secondary antibodies, respectively, and merged images are shown to visualize the colocalized (yellow) cells. Representative images (n = 10) are shown (bottom bar graph). Nikon NIS elementR was used to count the double positive cells, and mean ± SD values are shown as bar graph for the comparative average double positive cells for the indicated groups of mice. * and ** denotes p < 0.04 in all groups.
Figure 10
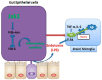
Model for Jak3 signaling in gut–brain axis. Conserved Jak3-mediated signaling in intestinal epithelial cells and microglial cells in the brain are shown.
Similar articles
- Role of Janus Kinase 3 in Predisposition to Obesity-associated Metabolic Syndrome.
Mishra J, Verma RK, Alpini G, Meng F, Kumar N. Mishra J, et al. J Biol Chem. 2015 Dec 4;290(49):29301-12. doi: 10.1074/jbc.M115.670331. Epub 2015 Oct 8. J Biol Chem. 2015. PMID: 26451047 Free PMC article. - Intestinal breast cancer resistance protein (BCRP) requires Janus kinase 3 activity for drug efflux and barrier functions in obesity.
Mishra J, Simonsen R, Kumar N. Mishra J, et al. J Biol Chem. 2019 Nov 29;294(48):18337-18348. doi: 10.1074/jbc.RA119.007758. Epub 2019 Oct 25. J Biol Chem. 2019. PMID: 31653704 Free PMC article. - Microbial lipopolysaccharide-induced inflammation contributes to cognitive impairment and white matter lesion progression in diet-induced obese mice with chronic cerebral hypoperfusion.
Inaba T, Yamashiro K, Kurita N, Ueno Y, Miyamoto N, Hira K, Nakajima S, Kijima C, Nakaguro R, Urabe T, Hattori N. Inaba T, et al. CNS Neurosci Ther. 2023 Jun;29 Suppl 1(Suppl 1):200-212. doi: 10.1111/cns.14301. Epub 2023 Jun 8. CNS Neurosci Ther. 2023. PMID: 37287396 Free PMC article. - Mucosal Epithelial Jak Kinases in Health and Diseases.
Kumar N, Kuang L, Villa R, Kumar P, Mishra J. Kumar N, et al. Mediators Inflamm. 2021 Mar 16;2021:6618924. doi: 10.1155/2021/6618924. eCollection 2021. Mediators Inflamm. 2021. PMID: 33814980 Free PMC article. Review. - Mitochondrial function in intestinal epithelium homeostasis and modulation in diet-induced obesity.
Guerbette T, Boudry G, Lan A. Guerbette T, et al. Mol Metab. 2022 Sep;63:101546. doi: 10.1016/j.molmet.2022.101546. Epub 2022 Jul 8. Mol Metab. 2022. PMID: 35817394 Free PMC article. Review.
Cited by
- Cognitive impairment and the gut-brain axis during 2014-2023: a bibliometric analysis.
He J, Wu J, Liu J, Wu H, Hua H. He J, et al. Front Neurol. 2024 Jul 5;15:1407956. doi: 10.3389/fneur.2024.1407956. eCollection 2024. Front Neurol. 2024. PMID: 39036641 Free PMC article. - Therapeutic Candidates for Alzheimer's Disease: Saponins.
Zhang R, Zeng M, Zhang X, Zheng Y, Lv N, Wang L, Gan J, Li Y, Jiang X, Yang L. Zhang R, et al. Int J Mol Sci. 2023 Jun 22;24(13):10505. doi: 10.3390/ijms241310505. Int J Mol Sci. 2023. PMID: 37445682 Free PMC article. Review. - The genetic advantage of healthy centenarians: unraveling the central role of NLRP3 in exceptional healthspan.
Verlinden SF. Verlinden SF. Front Aging. 2024 Sep 5;5:1452453. doi: 10.3389/fragi.2024.1452453. eCollection 2024. Front Aging. 2024. PMID: 39301197 Free PMC article. Review.
References
- Fiest K.M., Roberts J.I., Maxwell C.J., Hogan D., Smith E.E., Frolkis A., Cohen A., Kirk A., Pearson D., Pringsheim T., et al. The Prevalence and Incidence of Dementia Due to Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2016;43:S51–S82. doi: 10.1017/cjn.2016.36. - DOI - PubMed
- Weuve J., Barnes L.L., de Leon C.F.M., Rajan K.B., Beck T., Aggarwal N.T., Hebert L.E., Bennett D.A., Wilson R.S., Evans D.A. Cognitive aging in black and white Americans: Cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology. 2017;29:151–159. doi: 10.1097/EDE.0000000000000747. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials