GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules - PubMed (original) (raw)
GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules
E Kim et al. J Cell Biol. 1997.
Abstract
The molecular mechanisms underlying the organization of ion channels and signaling molecules at the synaptic junction are largely unknown. Recently, members of the PSD-95/SAP90 family of synaptic MAGUK (membrane-associated guanylate kinase) proteins have been shown to interact, via their NH2-terminal PDZ domains, with certain ion channels (NMDA receptors and K+ channels), thereby promoting the clustering of these proteins. Although the function of the NH2-terminal PDZ domains is relatively well characterized, the function of the Src homology 3 (SH3) domain and the guanylate kinase-like (GK) domain in the COOH-terminal half of PSD-95 has remained obscure. We now report the isolation of a novel synaptic protein, termed GKAP for guanylate kinase-associated protein, that binds directly to the GK domain of the four known members of the mammalian PSD-95 family. GKAP shows a unique domain structure and appears to be a major constituent of the postsynaptic density. GKAP colocalizes and coimmunoprecipitates with PSD-95 in vivo, and coclusters with PSD-95 and K+ channels/NMDA receptors in heterologous cells. Given their apparent lack of guanylate kinase enzymatic activity, the fact that the GK domain can act as a site for protein-protein interaction has implications for the function of diverse GK-containing proteins (such as p55, ZO-1, and LIN-2/CASK).
Figures
Figure 1
Primary structure of GKAP. (A) Rat brain cDNA clones isolated from the yeast two-hybrid screen using PSD-95 GK domain as bait are shown (black lines) aligned below a schematic of the domain organization of GKAP protein (drawn to scale). Numbers in parentheses refer to the number of times each clone was isolated from the yeast two-hybrid screen. White boxes indicate the presence of alternative inserts at three sites in GKAP, presumably due to alternative splicing (A, B, C, and D; 1, 2; X, Y; indicate different sequences found at these sites). Five 14 aa repeats (black) and the GKAP homology domain 1 (GH1, gray) are represented by boxes. The hGKAP cDNA clone contains a full-length human GKAP coding region and was obtained as an EST (accession No. Z45015, IMAGE consortium). GKAP cDNA clones 2.18, 2.6 and 2.2 contain different lengths of 3′ untranslated region (not shown), but share a common protein coding sequence. (B) Amino acid sequence alignment of rat and human GKAP (Genbank accession numbers U67987 and U67988, respectively). The rat sequence shown is that of GKAP clone 2.18. (R1-R5: 14 aa repeats; GH1: GKAP homology domain 1; ALT, alternative sequence variations or insertions due to presumed alternative splicing). Identical amino acid residues between rat and human sequences are shown in black boxes. Proline-rich motifs that are possible binding sequence for the SH3 domain are underlined.
Figure 2
GKAP interacts specifically with GK domains from members of the PSD-95 family. (A) GK domains from PSD-95, SAP97, chapsyn-110, and ZO-1 were tested for their binding to GKAP in the yeast two-hybrid assay, based on induction of yeast reporter genes HIS3 and β-galactosidase. HIS3 activity (measured by % of colonies growing on histidine-lacking medium): +++ (>60%), ++ (30–60%), + (10–30%), − (no significant growth); β-gal: (time taken for yeast colonies to turn blue in X-gal filter lift assays at room temperature): +++ (<45 min), ++ (45–90 min), + (90–240 min), − (no significant β-gal activity). (B) Results of reverse yeast two-hybrid screening of rat and human brain cDNA libraries using GKAP (clone 2.18) as bait. 9 of 10 GKAP-interacting clones contained the GK domains of one of the four known PSD-95 family members. These interacting clones are shown aligned below the a schematic diagram of the corresponding full-length protein. r, or h, indicates rat or human cDNA. Highlighted are: PDZ domains (black), SH3 domains (gray), GK domains (hatched), alternatively spliced insertions (open box). Numbers refer to amino acid residues at the boundaries of the cDNA clones and highlighted domains.
Figure 3
Minimal domains required for binding between PSD-95 GK domain and GKAP. (A) Various deletion variants of the GK domain of PSD-95 are shown as black lines aligned below the COOH-terminal region of PSD-95 in which the GK domain is represented by hatched box (aa 534-712). Each GK deletion variant was tested for interaction with GKAP clone 2.18 in the yeast two-hybrid system. Numbers refer to the amino acid residues that define the boundaries of each construct. HIS3 and β-gal reporter gene induction was measured as in Fig. 2. (B) Deletion variants of GKAP are shown schematically as black lines aligned below the NH2-terminal region of GKAP. GKAP deletion variants were tested for interaction with PSD-95 GK domain (aa 524-724) as in A.
Figure 4
Direct GK-GKAP binding in overlay filter binding assays. (A) GST-fusion proteins containing no insert (GST), or different regions of PSD-95 (PDZ1-2, SH3, or the GK domain), or GK domains of SAP97 and chapsyn-110, were separated by SDSPAGE, transferred to a nitrocellulose and probed with TrxGKAP (thioredoxin fusion protein of GKAP clone 2.1) (upper panel). Bound GKAP was visualized with anti-Trx antibody. After stripping, the same membrane was reprobed with anti-GST antibody to show the relative positions and amounts of the various target GST fusion proteins (lower panel). GKAP binds specifically to GK domains from PSD-95, SAP97, and chapsyn-110, but not to PDZ1-2, SH3 domains of PSD-95 or to GST alone. Positions of size markers are indicated in kD. (B) H6 fusion proteins of NH2- and COOH-terminal regions of GKAP (top diagram) were separated by SDS-PAGE, transferred to nitrocellulose, and probed with GST-GK fusions of PSD-95, SAP97, chapsyn-110, or with GST alone, as indicated (middle panels). Bound GK was visualized with anti-GST antibody, followed by stripping and reprobing with anti-T7-Tag antibody to show the relative positions and amounts of target GKAP fusion proteins on the membrane (bottom panels). The different GK domains, but not GST alone, bind specifically to the NH2-terminal region of GKAP that contains the 14 aa repeats (black boxes).
Figure 5
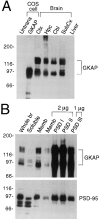
Expression pattern of GKAP protein in rat brain. (A) Specificity of GKAP antibodies and differential regional expression of GKAP in rat brain. Whole cell extracts of untransfected COS-7 cells (Untrans.), or of COS cells transfected with GKAP cDNA, were analyzed by immunoblotting with GKAP2.1 antibodies, along with membrane fractions (10 μg protein) from different regions of brain or liver, as indicated. Ctx (cortex), Hpc (hippocampus), Cbl (cerebellum), Subcx (subcortical regions). Positions of molecular size markers are shown in kD. (B) Immunoblot analysis of subcellular fractionation of GKAP. Lanes were loaded with rat brain fractions, as follows: Whole br (total brain homogenate, 20 μg protein), Soluble (S100 supernatant fraction of brain homogenate, 30 μg). Memb (crude synaptosomal membrane fraction, 10 μg or 2 μg, as indicated); PSDI, PSDII, and PSDIII (purified PSD fractions after extraction with Triton X-100 once [I], twice [II], or with Triton X-100 followed by sarkosyl [III]). Filters were probed with GKAP and PSD-95 antibodies, as indicated. (Equal percentages [rather than equal mass] of membrane and soluble fractions were loaded—the soluble fraction contained three times higher concentration of total protein than the membrane fraction. To show relative purification in the PSD fractions, only 2 μg of PSDI, PSDII, and 1 μg of PSDIII were immunoblotted and compared with 2 μg of synaptosomal membrane fraction.)
Figure 6
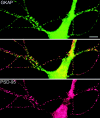
Colocalization of GKAP and PSD-95 in cultured hippocampal neurons. Double immunofluorescence labeling of neurons with rabbit anti-GKAP (GKAP2.1) antibodies (green, top panel), and with guinea pig anti-PSD-95 antibodies (red, bottom panel). Middle panel shows superposition of the two images. GKAP and PSD-95 immunoreactivities are colocalized (yellow) in a punctate pattern along dendrites. Bar, 10 μm.
Figure 7
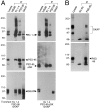
Coimmunoprecipitation of PSD95 and GKAP from cotransfected COS cells and from rat brain. (A) Extracts from COS-7 cells triply transfected with either Kv1.4 + PSD-95 + GKAP (left), or with Kv1.4 + PSD-95ΔGK + GKAP (right), were immunoprecipitated with Kv1.4, PSD-95, GKAP, or negative control NR2B antibodies, as indicated. Immunoprecipitates were then immunoblotted for Kv1.4, PSD-95, and GKAP, as indicated. First lane (lysate) was loaded directly with the transfected cell lysate (5% of input). (B) Extracts of rat cerebral cortex synaptosomal membranes were immunoprecipitated with PSD-95 antibodies, or with no primary antibodies. Immunoprecipitates were then immunoblotted with GKAP and PSD-95 antibodies as indicated. First lane (lysate) was loaded with detergent extract of rat cerebral cortex used for the immunoprecipitation (5% of input).
Figure 8
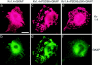
Recruitment of GKAP into Kv1.4/PSD-95 coclusters in COS cells, studied by double immunofluorescence labeling. In cells cotransfected with Kv1.4 and GKAP, (a and b), both Kv1.4 (a, red) and GKAP (b, green) are mainly distributed in a diffuse intracellular reticular pattern with perinuclear accumulation, with no evidence of interaction. In cells triply transfected with Kv1.4 + PSD-95 + GKAP (c and d), GKAP (d) now colocalized in clusters with Kv1.4 (c) and PSD-95 (data not shown). However, in cells triply transfected with Kv1.4 + PSD-95ΔGK + GKAP (e and f), Kv1.4 is still found in clusters (e) but GKAP no longer colocalizes with Kv1.4 and is instead diffusely distributed in the cell (f). The Kv1.4 clusters shown here are identical in nature to those formed with coexpression of Kv1.4 + PSD-95 in the absence of GKAP. Kv1.4 was visualized by rabbit anti-Kv1.4 antibodies and Cy3-conjugated anti–rabbit secondary antibodies. A myc-epitope tagged GKAP construct was used in these experiments to allow visualization of GKAP using mouse anti-myc monoclonal antibodies (9E10) and FITC-conjugated anti–mouse secondary antibodies. Bar, 5 μm.
Similar articles
- An intramolecular interaction between Src homology 3 domain and guanylate kinase-like domain required for channel clustering by postsynaptic density-95/SAP90.
Shin H, Hsueh YP, Yang FC, Kim E, Sheng M. Shin H, et al. J Neurosci. 2000 May 15;20(10):3580-7. doi: 10.1523/JNEUROSCI.20-10-03580.2000. J Neurosci. 2000. PMID: 10804199 Free PMC article. - SAPAPs. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density.
Takeuchi M, Hata Y, Hirao K, Toyoda A, Irie M, Takai Y. Takeuchi M, et al. J Biol Chem. 1997 May 2;272(18):11943-51. doi: 10.1074/jbc.272.18.11943. J Biol Chem. 1997. PMID: 9115257 - Functional analysis of the nucleotide binding domain of membrane-associated guanylate kinases.
Olsen O, Bredt DS. Olsen O, et al. J Biol Chem. 2003 Feb 28;278(9):6873-8. doi: 10.1074/jbc.M210165200. Epub 2002 Dec 12. J Biol Chem. 2003. PMID: 12482754 - PSD-95-like membrane associated guanylate kinases (PSD-MAGUKs) and synaptic plasticity.
Xu W. Xu W. Curr Opin Neurobiol. 2011 Apr;21(2):306-12. doi: 10.1016/j.conb.2011.03.001. Epub 2011 Mar 28. Curr Opin Neurobiol. 2011. PMID: 21450454 Free PMC article. Review. - MAGUKs: multifaceted synaptic organizers.
Won S, Levy JM, Nicoll RA, Roche KW. Won S, et al. Curr Opin Neurobiol. 2017 Apr;43:94-101. doi: 10.1016/j.conb.2017.01.006. Epub 2017 Feb 23. Curr Opin Neurobiol. 2017. PMID: 28236779 Free PMC article. Review.
Cited by
- Structural properties and peptide ligand binding of the capsid homology domains of human Arc.
Hallin EI, Bramham CR, Kursula P. Hallin EI, et al. Biochem Biophys Rep. 2021 Mar 5;26:100975. doi: 10.1016/j.bbrep.2021.100975. eCollection 2021 Jul. Biochem Biophys Rep. 2021. PMID: 33732907 Free PMC article. - A perspective on molecular signalling dysfunction, its clinical relevance and therapeutics in autism spectrum disorder.
Purushotham SS, Reddy NMN, D'Souza MN, Choudhury NR, Ganguly A, Gopalakrishna N, Muddashetty R, Clement JP. Purushotham SS, et al. Exp Brain Res. 2022 Oct;240(10):2525-2567. doi: 10.1007/s00221-022-06448-x. Epub 2022 Sep 5. Exp Brain Res. 2022. PMID: 36063192 Review. - GKAP orchestrates activity-dependent postsynaptic protein remodeling and homeostatic scaling.
Shin SM, Zhang N, Hansen J, Gerges NZ, Pak DT, Sheng M, Lee SH. Shin SM, et al. Nat Neurosci. 2012 Dec;15(12):1655-66. doi: 10.1038/nn.3259. Epub 2012 Nov 11. Nat Neurosci. 2012. PMID: 23143515 Free PMC article. - Hyperfunction of post-synaptic density protein 95 promotes seizure response in early-stage aβ pathology.
Yook Y, Lee KY, Kim E, Lizarazo S, Yu X, Tsai NP. Yook Y, et al. EMBO Rep. 2024 Mar;25(3):1233-1255. doi: 10.1038/s44319-024-00090-0. Epub 2024 Feb 27. EMBO Rep. 2024. PMID: 38413732 Free PMC article. - Synaptic targeting and localization of discs-large is a stepwise process controlled by different domains of the protein.
Thomas U, Ebitsch S, Gorczyca M, Koh YH, Hough CD, Woods D, Gundelfinger ED, Budnik V. Thomas U, et al. Curr Biol. 2000 Sep 21;10(18):1108-17. doi: 10.1016/s0960-9822(00)00696-5. Curr Biol. 2000. PMID: 10996791 Free PMC article.
References
- Anderson JM. Cell signalling-MAGUK magic. Curr Biol. 1996;6:382–384. - PubMed
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–425. - PubMed
- Bartel, P.L., C.-T. Chien, R. Sternglanz, and S. Fields. 1993. Using the 2-hybrid system to detect protein-protein interactions. In Cellular Interactions in Development: A Practical Approach, Oxford University Press, Oxford. 153– 179.
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. - PubMed
- Cho K-O, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous