Activity-dependent expression of NT-3 in muscle cells in culture: implications in the development of neuromuscular junctions - PubMed (original) (raw)
Activity-dependent expression of NT-3 in muscle cells in culture: implications in the development of neuromuscular junctions
K Xie et al. J Neurosci. 1997.
Abstract
Although activity-dependent expression of neurotrophins has been studied extensively in the CNS, its physiological role during synapse development is not well established. At the developing neuromuscular junction in culture, exogenous application of the neurotrophin BDNF or NT-3 has been shown to acutely potentiate synaptic transmission and chronically promote synapse maturation. Using the same cell culture model, we have investigated activity-dependent neurotrophin expression in muscle cells and its role in developing neuromuscular synapses. Membrane depolarization, elicited by either depolarizing agents or repetitive electric stimulation, rapidly and specifically increased the levels of NT-3 mRNA in developing Xenopus laevis muscle cells in culture. NT-3 gene expression also was enhanced by acetylcholine (ACh), the neurotransmitter that causes muscle membrane depolarization. The effects of depolarization were mediated by increasing intracellular calcium concentration. Moreover, factor(s) induced by membrane depolarization appeared to enhance synaptic transmission at the developing neuromuscular junction. The frequency of spontaneous synaptic currents (SSCs) recorded from neuromuscular synapses was increased significantly after treatment with conditioned medium from depolarized muscle cultures. The amplitude, rise time, and decay time of SSCs were not affected, indicating a presynaptic action of the conditioned medium. The effects of the conditioned medium were blocked, partially, by the NT-3 scavenger TrkC-IgG, suggesting that the potentiation of synaptic efficacy was attributable, at least in part, to elevated NT-3 as a consequence of muscle depolarization. Thus, activity-dependent expression of muscle NT-3 may contribute to the development of the neuromuscular synapse.
Figures
Fig. 1.
Nucleotide and amino acid sequence of_Xenopus_ NT-3. The deduced amino acid sequence, in_single-letter code_, is shown below the nucleotide sequence. Primers used for cloning are_underlined_ (in the order of appearance: HNT 3, XNT 5, XNT 4, and HNT 6). Primers used for the quantitative RT-PCR assay are_boxed_ (NT-3-1 and NT-3-2).
Fig. 2.
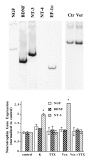
Effect of membrane depolarization on neurotrophin gene expression in Xenopus embryonic myocytes cultured for 3 d. Top, Left, A representative gel showing the expression of neurotrophin genes in these cells. The PCR products (cycle numbers: EF-1α, 24; all neurotrophins, 34) were separated on a 6% acrylamide gel and viewed by phosphoimaging. The PCR products exhibited predicted sizes for the respective neurotrophins (NGF, 522 bp;BDNF, 377 bp; NT-3, 402 bp). Although_NT-4_ was not detectable in this experiment with the use of embryonic muscle cells, similar PCR that used mRNA from adult leg muscle exhibited the predicted 548 bp band. Top,Right, A representative gel showing the effect of depolarization on muscle NT-3 mRNA expression. Myocytes were cultured for 3 d and treated with (Ver) or without (Ctr) veratridine (10 μ
m
) for 2 hr. RNA was extracted from each of these cultures and processed for RT-PCR assay. Note that the signals for NT-3 were normalized to that for EF-1α. Bottom, Summary of the effect of membrane depolarization on neurotrophin gene expression in embryonic myocytes. Six such experiments were performed, and all generated similar results. This figure shows results from one such experiment.Xenopus muscle cells from stage 22 embryos were cultured for 3 d and then treated with the indicated drugs for 1 hr.K, KCl, 35 m
m
; TTX, tetrodotoxin, 1 μ
m
; Ver, veratridine, 10 μ
m
; control, no drug treatment. Each group contains cultures in triplicate. RNA was extracted from each of these cultures and processed for RT-PCR assay. The signal of neurotrophin mRNA from each of these cultures was normalized to that of EF-1α mRNA from the same culture. The signals for each condition were averaged. Then the relative levels of neurotrophin mRNAs were obtained by normalizing data from all experimental conditions to control values. In this and all other figures, error bars are SD. *Significantly different from control (p < 0.001, two-tailed Student’s t test).
Fig. 3.
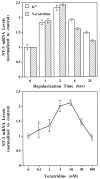
Characterization of depolarization-induced NT-3 gene expression. Embryonic Xenopus muscle cells were cultured for 3 d and then treated with the depolarizing agents veratridine or K+ and harvested for NT-3 mRNA measurement with RT-PCR assay, as described in Figure 2. Top, Time course of NT-3 gene expression induced by depolarization. KCl (35 m
m
) or veratridine (10 μ
m
) was applied to the cultures at time 0 (control). The muscle cells were harvested at different time points after depolarization. The experiments were performed three times (n = 3), and the results were virtually identical. A typical result is shown.Bottom, Concentration dependence of veratridine effects. The muscle cultures were treated for 2 hr with different concentrations of veratridine, as indicated. Control, no veratridine treatment;n = 3.
Fig. 4.
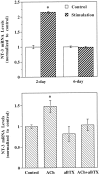
Effects of electric stimulation and ACh on the levels of muscle NT-3 mRNA. Top, Effects of electrical stimulation. Xenopus muscle cultures of different developmental stages (2-day and 6-day) were stimulated at 6 Hz via agarose bridges for 1 hr. Stimulation intensity was adjusted to be just above the threshold of muscle contraction. Control, No electrical stimulation;n = 3. Bottom, Effects of ACh. Muscle cells cultured for 3 d were treated with ACh(0.1 m
m
), α-bungarotoxin (aBTX, 10 μg/ml), or both, as indicated. Drugs and Ringer’s solution were perfused to muscle cultures alternately (5 min/10 min) for a total of 2 hr. Control, No drug treatment; n = 3.
Fig. 5.
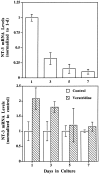
Developmental regulation of NT-3 genes.Top, NT-3 gene expression during development in culture. Muscle cells cultured for different lengths of time, as indicated, were harvested, and the relative levels of NT-3 mRNA were measured (normalized to 1 d in culture); n = 3.Bottom, Effects of depolarization on NT-3 gene expression at different developmental stages. The same muscle cultures as above were treated with or without veratridine (10 μ
m
) for 2 hr, and NT-3 gene expression was determined. Data from veratridine-treated cultures were normalized to their sister control (untreated) cultures for clarity of presentation; _n_= 3.
Fig. 6.
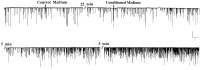
Potentiation of synaptic activity at the developing neuromuscular junction by conditioned medium from depolarized muscle cells cultured for 3 d. A representative recording of spontaneous synaptic currents (SSCs) from an innervated myocyte in a 1-d-old nerve–muscle culture before and after bath application of control and conditioned media is shown. Downward deflections are SSCs (_V_h = −70 mV, filtered at 150 Hz). Calibration: 250 pA, 20 sec. Conditioned Medium was the supernatant collected from a muscle culture treated with veratridine (10 μ
m
) for 2 hr, followed by removal of veratridine via Centricon dialysis. The volume of conditioned medium added to the recording dish was 200 μl, which is the equivalent of the supernatant from two muscle cultures.Control Medium was prepared from cultures in the same way as Conditioned Medium except without veratridine treatment.
Fig. 7.
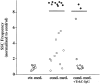
Quantitation of changes in synaptic activity under different experimental conditions. The frequency of SSCs was calculated as the number of SSC events per minute, averaged from at least 10 min of recording in each condition. Each _data point_represents one experiment. The ratios of SSC frequencies at 1 min before and 20 min after the control medium (ctr.med.) or conditioned medium (cond.med.) are presented. In the cond. med. + TrkC-IgG group, conditioned medium was added to cultures pretreated with TrkC-IgG (1 μg/ml) for 0.5–2 hr. The filled circles and filled diamonds represent the ratio of SSC frequency higher than 10.
Fig. 8.
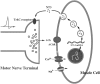
A schematic model for reciprocal interaction of synaptic activity and NT-3 expression during synapse formation. During neuromuscular development presynaptic activity and consequent depolarization of the postsynaptic myocyte cause a specific increase in the levels of NT-3, which in turn feeds back on presynaptic terminals. Activation of presynaptic TrkC receptors potentiates synaptic transmission and promotes synaptic maturation.
Similar articles
- Neurotrophins promote maturation of developing neuromuscular synapses.
Wang T, Xie K, Lu B. Wang T, et al. J Neurosci. 1995 Jul;15(7 Pt 1):4796-805. doi: 10.1523/JNEUROSCI.15-07-04796.1995. J Neurosci. 1995. PMID: 7623111 Free PMC article. - Regulation of quantal secretion by neurotrophic factors at developing motoneurons in Xenopus cell cultures.
Liou JC, Yang RS, Fu WM. Liou JC, et al. J Physiol. 1997 Aug 15;503 ( Pt 1)(Pt 1):129-39. doi: 10.1111/j.1469-7793.1997.129bi.x. J Physiol. 1997. PMID: 9288681 Free PMC article. - Activity-dependent modulation of developing neuromuscular synapses.
Poo MM. Poo MM. Adv Second Messenger Phosphoprotein Res. 1994;29:521-7. doi: 10.1016/s1040-7952(06)80033-9. Adv Second Messenger Phosphoprotein Res. 1994. PMID: 7848730 Review. - Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS.
Lessmann V. Lessmann V. Gen Pharmacol. 1998 Nov;31(5):667-74. doi: 10.1016/s0306-3623(98)00190-6. Gen Pharmacol. 1998. PMID: 9809461 Review.
Cited by
- Eif4a3 is required for accurate splicing of the Xenopus laevis ryanodine receptor pre-mRNA.
Haremaki T, Weinstein DC. Haremaki T, et al. Dev Biol. 2012 Dec 1;372(1):103-10. doi: 10.1016/j.ydbio.2012.08.013. Epub 2012 Aug 28. Dev Biol. 2012. PMID: 22944195 Free PMC article. - The development of local, layer-specific visual cortical axons in the absence of extrinsic influences and intrinsic activity.
Dantzker JL, Callaway EM. Dantzker JL, et al. J Neurosci. 1998 Jun 1;18(11):4145-54. doi: 10.1523/JNEUROSCI.18-11-04145.1998. J Neurosci. 1998. PMID: 9592094 Free PMC article. - Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons.
Vicario-Abejón C, Collin C, McKay RD, Segal M. Vicario-Abejón C, et al. J Neurosci. 1998 Sep 15;18(18):7256-71. doi: 10.1523/JNEUROSCI.18-18-07256.1998. J Neurosci. 1998. PMID: 9736647 Free PMC article. - Muscle spindle-derived neurotrophin 3 regulates synaptic connectivity between muscle sensory and motor neurons.
Chen HH, Tourtellotte WG, Frank E. Chen HH, et al. J Neurosci. 2002 May 1;22(9):3512-9. doi: 10.1523/JNEUROSCI.22-09-03512.2002. J Neurosci. 2002. PMID: 11978828 Free PMC article. - Differential regulation of synaptic vesicle protein genes by target and synaptic activity.
Plunkett JA, Baccus SA, Bixby JL. Plunkett JA, et al. J Neurosci. 1998 Aug 1;18(15):5832-8. doi: 10.1523/JNEUROSCI.18-15-05832.1998. J Neurosci. 1998. PMID: 9671670 Free PMC article.
References
- Barbacid M. Nerve growth factor: a tale of two receptors. Oncogene. 1993;8:2033–2042. - PubMed
- Barde Y. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. - PubMed
- Cabelli RJ, Horn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267:1662–1666. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials