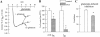Positive and negative coupling of the metabotropic glutamate receptors to a G protein-activated K+ channel, GIRK, in Xenopus oocytes - PubMed (original) (raw)
Positive and negative coupling of the metabotropic glutamate receptors to a G protein-activated K+ channel, GIRK, in Xenopus oocytes
D Sharon et al. J Gen Physiol. 1997 Apr.
Abstract
Metabotropic glutamate receptors (mGluRs) control intracellular signaling cascades through activation of G proteins. The inwardly rectifying K+ channel, GIRK, is activated by the beta gamma subunits of G proteins and is widely expressed in the brain. We investigated whether an interaction between mGluRs and GIRK is possible, using Xenopus oocytes expressing mGluRs and a cardiac/brain subunit of GIRK, GIRK1, with or without another brain subunit, GIRK2. mGluRs known to inhibit adenylyl cyclase (types 2, 3, 4, 6, and 7) activated the GIRK channel. The strongest response was observed with mGluR2; it was inhibited by pertussis toxin (PTX). This is consistent with the activation of GIRK by Gi/Go-coupled receptors. In contrast, mGluR1a and mGluR5 receptors known to activate phospholipase C, presumably via G proteins of the Gq class, inhibited the channel's activity. The inhibition was preceded by an initial weak activation, which was more prominent at higher levels of mGluR1a expression. The inhibition of GIRK activity by mGluR1a was suppressed by a broad-specificity protein kinase inhibitor, staurosporine, and by a specific protein kinase C (PKC) inhibitor, bis-indolylmaleimide, but not by PTX, Ca(2-)chelation, or calphostin C. Thus, mGluR1a inhibits the GIRK channel primarily via a pathway involving activation of a PTX-insensitive G protein and, eventually, of a subtype of PKC, possibly PKC-mu. In contrast, the initial activation of GIRK1 caused by mGluR1a was suppressed by PTX but not by the protein kinase inhibitors. Thus, this activation probably results from a promiscuous coupling of mGluR1a to a Gi/Go protein. The observed modulations may be involved in the mGluRs effects on neuronal excitability in the brain. Inhibition of GIRK by phospholipase C-activating mGluRs bears upon the problem of specificity of G protein (GIRK interaction) helping to explain why receptors coupled to Gq are inefficient in activating GIRK.
Figures
Figure 4
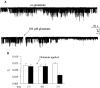
Glutamate applied outside the patch pipette blocks GIRK activity in cell- attached patch clamp recordings from cells expressing mGluR1a, GIRK1, Gβ1, and Gγ2. (A) Top : a trace from a cell unexposed to glutamate, which shows steady activity of GIRK1. Bottom: a trace from a cell exposed to glutamate at the time indicated by the arrow, showing inhibition of channel activity. The time period during which glutamate was added to the bath is blanked out. (B) Glutamate reduces open probability (Po) in cells exposed to glutamate. Filled bars, Po in cells exposed to glutamate (n = 5). Empty bars, Po in cells unexposed to glutamate (n = 5). Po was averaged over periods of 3 min. The abscissa shows time after the start of the record. Glutamate was added at t = 3 min (arrow).
Figure 1
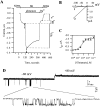
GIRK activation by mGluR2. (A) Two-electrode voltage-clamp recording from a cell expressing mGluR2 and GIRK1. Bars above trace show solutions applied. (B) Current-voltage relationships of the GIRK currents at the times indicated by arrows in A: in hK alone (1) and in glutamate (2). Each net GIRK1 I-V curve was obtained by subtraction of the I-V curve recorded after addition of Ba2+. (C) Gluta-mate dose-response curve, with apparent K d = 0.63 μM, fitted to the standard Michaelis-Menten equation. n = 3 or 4 cells for each glutamate concentration. (D) A cell-attached patch clamp record of the activity of the GIRK channel with 10 μM K-glutamate in the pipette, from an oocyte injected with RNAs of GIRK1 and mGluR2.
Figure 2
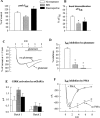
Inhibition of GIRK by mGluR1a. (A) Two-electrode voltage-clamp recording from a single cell injected with RNAs of GIRK1 (500 pg) and mGluR1a (25 pg). ND, stands for ND96. (B) Average recording from cells of one donor exposed to glutamate (•, n = 4) and unexposed to glutamate (○, n = 6). Ba2+ was not applied, and current was expressed as percent of the current in hK solution (IhK + In); I = 0 in ND96. (C) Inhibition of GIRK channel activity by mGluR1a in EGTA-treated oocytes: comparison of cells exposed to glutamate (n = 4) to cells unexposed to glutamate (n = 4). The cells were from the same donor, injected with 25 pg mGluR1a RNA and 500 pg GIRK1 RNA. The ND96 solution was changed to the hK solution at time t = 0. Currents in each oocyte were normalized to the peak basal GIRK current, IhK. Average traces from all 4 oocytes are shown. The mean IhK was 313 ± 29 nA (n = 8) in these oocytes. (D) Current-voltage relationships of GIRK currents at the times indicated by arrows in C, in a cell injected with EGTA: in hK (1) and in glutamate after the decay of the current (2). Net GIRK I-V curves were obtained as in Fig. 1_B_. (E) Inhibition of GIRK1/GIRK2 channel activity by mGluR1a in EGTA-treated oocytes. Presentation as in C; n = 4 oocytes for each treatment. In this batch, oocytes were injected with 50 pg mGluR1a RNA and 250 pg of each of the GIRK1 and GIRK2 RNAs. Average IhK was 4,126 ± 707 nA (n = 8). (F) Absence of glutamate-activated inward current in oocytes incubated in 300 μM BaCl2. Oocytes were injected with RNAs of mGluR1a (25 pg) and GIRK1 (500 pg) and treated with EGTA. Ba2+ (300 μM) was added to all solutions. Currents in each cell were normalized to the peak inward current observed in the hK solution, which, because of the block of GIRK, equals to In (the average current in the hK solution was 86 ± 8 nA, n = 5). (G) Inhibition of m2R-induced activation of GIRK by mGluR1a. Oocytes expressing mGluR1a, m2R, and GIRK1, and injected with EGTA before recording, were placed in hK solution and then exposed to 10 μM ACh, which activated GIRK (the record starts at the time of addition of ACh). This current is denoted IACh. 3 min after exposure to ACh (at the time indicated by the arrow), one group of cells was exposed to glutamate (•, n = 3), which caused a decrease in the current compared with cells that were not exposed to glutamate (○, n = 3). The current is expressed as percent of IACh; records averaged from all oocytes in each group are shown.
Figure 5
mGluR1a-induced inhibition of GIRK is blocked by PKC inhibitors and mimicked by PMA. Oocytes expressing mGluR1a (30 or 50 pg/ oocyte), GIRK1 and GIRK2 (250 or 500 pg/oocyte) were injected with EGTA before recording (except in F). In A, B, D and E, the treatments are presented as follows: empty bars, no treatment (vehicle only); shaded bars, BIS; black bars, staurosporine. (A) Staurosporine and BIS do not significantly alter the amplitude of IhK. Summary of two oocyte batches, n = 8 for each treatment. In each oocyte, IhK was normalized to the average IhK amplitude in untreated cells of the same oocyte batch. (B) Staurosporine and BIS attenuate the basal desensitization of IhK. Same oocytes as in A. In each oocyte, the extent of desensitization was calculated as [1 − (IhK at the end of the 8 min exposure to hK)/ (peak IhK)] × 100%. (C) Gluta-mate (trace denoted: + gluta-mate) fails to cause inhibition of GIRK in a representative cell treated with staurosporine; the activation of GIRK remains unimpaired. For comparison, a re-cord is shown from an oocyte (from the same donor) treated with staurosporine but not exposed to glutamate (no gluta-mate). (D) The mGluR1a- induced inhibition of IhK is suppressed by BIS and staurosporine: a summary of two experiments. Same oocytes batches as in A and B. The extent of IhK inhibition was calculated as explained in Fig. 3_C_. (E) mGluR1a-induced GIRK activation is not significantly changed by BIS and staurosporine. (F) PMA inhibits the basal GIRK current. Oocytes were incubated in ND96 as usual and, at t = 0, exposed either to the normal hK solution (no PMA; n = 5) or to hK containing 10 nM PMA (+ PMA; n = 5). Records averaged from all oocytes in each group are shown.
Figure 3
PTX suppresses mGluR1a-induced activation of GIRK but has little effect on mGluR1a-induced GIRK inhibition. (A) Inhibition of GIRK1/ GIRK2 channel activity by mGluR1a in EGTA-injected oocytes treated with PTX: comparison of cells exposed to glutamate (n = 4) to cells unexposed to glutamate (n = 4). The cells were injected with RNAs of mGluR1a (50 pg), GIRK1 (250 pg), and GIRK2 (250 pg). The ND96 solution was changed to the hK solution at time t = 0. Currents in each oocyte were normalized to the peak basal GIRK current, IhK. Records averaged from all oocytes in each group (n = 4) are shown. Average IhK was 833 ± 193 nA (n = 8) in these oocytes, and 1,993 ± 331 nA (n = 8) in PTX-untreated oocytes of the same donor. (B) The effect of PTX treatment on basal (IhK) and glutamate-evoked (Iglu) GIRK1/GIRK2 currents: a summary from oocytes of two donors (n = 8 in each group). IhK or Iglu in each cell was expressed as percent of control (i.e., average IhK or Iglu in the untreated group of oocytes of the same donor), and the results were averaged across all cells. Asterisks denote statistically significant differences, P < 0.05 or better. (C) The effect of PTX treatment on mGluR1a-induced GIRK1/GIRK2 inhibition. Same two oocyte batches as in B. The extent of glutamate-evoked inhibition was calculated as follows: first, in each cell, the current at the end of the record (t = 8 min in the hK solution) was expressed as percent of IhK measured at t = 2 min. In glutamate-exposed oocytes, this value was normalized to the average current in cells unexposed to glutamate at t = 8 min. This normalization procedure allowed averaging of the results despite the large difference in the absolute amplitudes of the currents recorded in oocytes of different donors.
Similar articles
- Metabotropic glutamate receptors activate G-protein-coupled inwardly rectifying potassium channels in Xenopus oocytes.
Saugstad JA, Segerson TP, Westbrook GL. Saugstad JA, et al. J Neurosci. 1996 Oct 1;16(19):5979-85. doi: 10.1523/JNEUROSCI.16-19-05979.1996. J Neurosci. 1996. PMID: 8815880 Free PMC article. - Activation and inhibition of neuronal G protein-gated inwardly rectifying K(+) channels by P2Y nucleotide receptors.
Filippov AK, Fernández-Fernández JM, Marsh SJ, Simon J, Barnard EA, Brown DA. Filippov AK, et al. Mol Pharmacol. 2004 Sep;66(3):468-77. doi: 10.1124/mol.66.3.. Mol Pharmacol. 2004. PMID: 15322238 - G-protein mediated gating of inward-rectifier K+ channels.
Mark MD, Herlitze S. Mark MD, et al. Eur J Biochem. 2000 Oct;267(19):5830-6. doi: 10.1046/j.1432-1327.2000.01670.x. Eur J Biochem. 2000. PMID: 10998041 Review. - G protein-coupled receptor signaling to Kir channels in Xenopus oocytes.
Hatcher-Solis C, Fribourg M, Spyridaki K, Younkin J, Ellaithy A, Xiang G, Liapakis G, Gonzalez-Maeso J, Zhang H, Cui M, Logothetis DE. Hatcher-Solis C, et al. Curr Pharm Biotechnol. 2014;15(10):987-95. doi: 10.2174/1389201015666141031111916. Curr Pharm Biotechnol. 2014. PMID: 25374032 Free PMC article. Review.
Cited by
- Group III metabotropic glutamate receptors: guardians against excitotoxicity in ischemic brain injury, with implications for neonatal contexts.
Mielecki D, Salińska E. Mielecki D, et al. Pharmacol Rep. 2024 Sep 17. doi: 10.1007/s43440-024-00651-z. Online ahead of print. Pharmacol Rep. 2024. PMID: 39298028 Review. - Metabotropic glutamate receptors-guardians and gatekeepers in neonatal hypoxic-ischemic brain injury.
Mielecki D, Bratek-Gerej E, Salińska E. Mielecki D, et al. Pharmacol Rep. 2024 Sep 17. doi: 10.1007/s43440-024-00653-x. Online ahead of print. Pharmacol Rep. 2024. PMID: 39289333 Review. - Psychedelic Targeting of Metabotropic Glutamate Receptor 2 and Its Implications for the Treatment of Alcoholism.
Domanegg K, Sommer WH, Meinhardt MW. Domanegg K, et al. Cells. 2023 Mar 22;12(6):963. doi: 10.3390/cells12060963. Cells. 2023. PMID: 36980303 Free PMC article. Review. - Glutamate Transporters EAAT2 and EAAT5 Differentially Shape Synaptic Transmission from Rod Bipolar Cell Terminals.
Tang FS, Yuan HL, Liu JB, Zhang G, Chen SY, Ke JB. Tang FS, et al. eNeuro. 2022 May 18;9(3):ENEURO.0074-22.2022. doi: 10.1523/ENEURO.0074-22.2022. Print 2022 May-Jun. eNeuro. 2022. PMID: 35523583 Free PMC article. - The Implication of Glial Metabotropic Glutamate Receptors in Alzheimer's Disease.
de Lima IBQ, Ribeiro FM. de Lima IBQ, et al. Curr Neuropharmacol. 2023;21(2):164-182. doi: 10.2174/1570159X20666211223140303. Curr Neuropharmacol. 2023. PMID: 34951388 Free PMC article. Review.
References
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+signal transduction. J Biol Chem. 1992;267:13361–13368. - PubMed
- Bausch SB, Patterson TA, Ehrengruber M, Lester HA, Davidson N, Chavkin C. Colocalization of μ opioid receptors with GIRK1 potassium channels in the rat brain: an immunohistochemical study. Recept Chann. 1995;3:221–241. - PubMed
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. - PubMed
- Berstein G, Blank JL, Smrcka AV, Higashijima T, Sternweis PC, Exton JH, Ross EM. Reconstitution of agonist-stimulated phosphatidylinositol 4,5 bisphosphate hydrolysis using purified m1 muscarinic receptor, Gq/11and phospholipase C-β1. J Biol Chem. 1992;267:8081–8088. - PubMed
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for βγ dimers as well as α subunits. Cell. 1992;71:1069–1072. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous