Cytokine manipulation of primitive human hematopoietic cell self-renewal - PubMed (original) (raw)
Comparative Study
Cytokine manipulation of primitive human hematopoietic cell self-renewal
P W Zandstra et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Previous studies have shown that primitive human hematopoietic cells detectable as long-term culture-initiating cells (LTC-ICs) and colony-forming cells (CFCs) can be amplified when CD34(+) CD38(-) marrow cells are cultured for 10 days in serum-free medium containing flt3 ligand (FL), Steel factor (SF), interleukin (IL)-3, IL-6, and granulocyte colony-stimulating factor. We now show that the generation of these two cell types in such cultures is differentially affected at the single cell level by changes in the concentrations of these cytokines. Thus, maximal expansion of LTC-ICs (60-fold) was obtained in the presence of 30 times more FL, SF, IL-3, IL-6, and granulocyte colony-stimulating factor than could concomitantly stimulate the near-maximal (280-fold) amplification of CFCs. Furthermore, the reduced ability of suboptimal cytokine concentrations to support the production of LTC-ICs could be ascribed to a differential response of the stimulated cells since this was not accompanied by a change in the number of input CD34(+) CD38(-) cells that proliferated. Reduced LTC-IC amplification in the absence of a significant effect on CFC generation also occurred when the concentrations of FL and SF were decreased but the concentration of IL-3 was high (as compared with cultures containing high levels of all three cytokines). To our knowledge, these findings provide the first evidence suggesting that extrinsically acting cytokines can alter the self-renewal behavior of primary human hematopoietic stem cells independent of effects on their viability or proliferation.
Figures
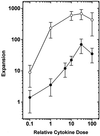
Figure 1
Expansion of LTC-IC (•) and CFC (○) numbers (relative to input) in 10-day 100-μl serum-free cultures initiated with 200 CD34+ CD38− cells. Input LTC-IC and CFC numbers were 8.2 ± 3.3 and 7.3 ± 2.6 per 100 CD34+ CD38− cells, respectively. A relative cytokine dose of 1 represents SF at 10 ng/ml, FL at 10 ng/ml, IL-3 at 2 ng/ml, IL-6 at 2 ng/ml, and G-CSF at 2 ng/ml. Points represent the mean ± SEM of data from three to seven experiments performed with three to five bone marrow samples.
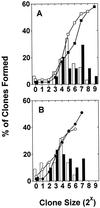
Figure 2
Size distribution (bars) and cumulative frequencies (symbols) of clones generated from a representative experiment in which single PI− AnnexinV− CD34+ CD38− cells were cultured for 10 days in serum-free medium supplemented with (i) FL at 300 ng/ml, SF at 300 ng/ml, IL-3 at 60 ng/ml, IL-6 at 60 ng/ml, and G-CSF at 60 ng/ml (solid bars and ▪, n = 59 wells) or (ii) FL at 30 ng/ml, SF at 30 ng/ml, IL-3 at 6 ng/ml, IL-6 at 6 ng/ml, and G-CSF at 6 ng/ml (open bars and □, n = 59 wells) (A) or (iii) FL at 300 ng/ml, SF at 300 ng/ml, and IL-3 at 60 ng/ml (solid bars, •, n = 59 wells) or (iv) FL at 10 ng/ml, SF at 10 ng/ml, and IL-3 at 60 ng/ml (open bars and ○, n = 57 wells) (B). At the end of the 10 days, all cells from all clones generated under a given set of conditions were pooled and assayed for their content of CFCs and LTC-ICs. The results of these assays confirmed the results shown in Fig. 1 (for the comparison undertaken in A) and in Table 1 (for the comparison undertaken in B) (see Results).
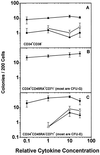
Figure 3
Number of colonies generated from 200 CD34+ CD38− (A), CD34+ CD45RA+ CD71− (B), or CD34+ CD45RA− CD71+ cells (C) plated directly in methylcellulose containing different concentrations of the same mixture of five cytokines used in the experiments shown in Fig. 1. Points for erythroid (•, from CFU-E and BFU-E), mixed (○, from CFU-GEMM), and myeloid (▴, from CFU-GM) colonies represent the mean ± SEM of values obtained from four experiments.
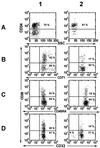
Figure 4
Representative flow cytometric analyses of CD34 expression vs. side scatter (SSC) (row A) and within the CD34+ cell population for expression of CD38 and CD71 (row B), CD45RA (row C), or CD33 (row D) on subpopulations of viable (PI−) cells generated from 200 input CD34+ CD38− Lin− cells after 10 days in serum-free cultures supplemented with either FL at 100 ng/ml, SF at 100 ng/ml, IL-3 at 20 ng/ml, IL-6 at 20 ng/ml, and G-CSF at 20 ng/ml (column 1) or FL at 300 ng/ml, SF at 300 ng/ml, and IL-3 at 60 ng/ml (column 2). The lower left-hand quadrant in each plot represents boundaries set by 99.9% of the unstained and isotype-labeled antibody controls.
Similar articles
- Differential cytokine effects on primitive (CD34+CD38-) human hematopoietic cells: novel responses to Flt3-ligand and thrombopoietin.
Petzer AL, Zandstra PW, Piret JM, Eaves CJ. Petzer AL, et al. J Exp Med. 1996 Jun 1;183(6):2551-8. doi: 10.1084/jem.183.6.2551. J Exp Med. 1996. PMID: 8676076 Free PMC article. - Murine stromal cells counteract the loss of long-term culture-initiating cell potential induced by cytokines in CD34(+)CD38(low/neg) human bone marrow cells.
Bennaceur-Griscelli A, Tourino C, Izac B, Vainchenker W, Coulombel L. Bennaceur-Griscelli A, et al. Blood. 1999 Jul 15;94(2):529-38. Blood. 1999. PMID: 10397720 - Humoral regulation of hematopoietic stem cells.
Ogawa M, Matsunaga T. Ogawa M, et al. Ann N Y Acad Sci. 1999 Apr 30;872:17-23; discussion 23-4. doi: 10.1111/j.1749-6632.1999.tb08449.x. Ann N Y Acad Sci. 1999. PMID: 10372107 Review. - Advances in hematopoietic stem cell culture.
Audet J, Zandstra PW, Eaves CJ, Piret JM. Audet J, et al. Curr Opin Biotechnol. 1998 Apr;9(2):146-51. doi: 10.1016/s0958-1669(98)80107-9. Curr Opin Biotechnol. 1998. PMID: 9588003 Review.
Cited by
- Chronic myelogenous leukemia stem and progenitor cells demonstrate chromosomal instability related to repeated breakage-fusion-bridge cycles mediated by increased nonhomologous end joining.
Chakraborty S, Stark JM, Sun CL, Modi H, Chen W, O'Connor TR, Forman SJ, Bhatia S, Bhatia R. Chakraborty S, et al. Blood. 2012 Jun 28;119(26):6187-97. doi: 10.1182/blood-2011-05-352252. Epub 2012 Apr 4. Blood. 2012. PMID: 22493298 Free PMC article. - An Overview on Human Umbilical Cord Blood Stem Cell-Based Alternative In Vitro Models for Developmental Neurotoxicity Assessment.
Singh AK, Kashyap MP. Singh AK, et al. Mol Neurobiol. 2016 Jul;53(5):3216-3226. doi: 10.1007/s12035-015-9202-6. Epub 2015 Jun 4. Mol Neurobiol. 2016. PMID: 26041658 Review. - Establishment and regulation of the HSC niche: Roles of osteoblastic and vascular compartments.
Coskun S, Hirschi KK. Coskun S, et al. Birth Defects Res C Embryo Today. 2010 Dec;90(4):229-42. doi: 10.1002/bdrc.20194. Birth Defects Res C Embryo Today. 2010. PMID: 21181885 Free PMC article. Review. - Distinct signaling programs control human hematopoietic stem cell survival and proliferation.
Knapp DJ, Hammond CA, Aghaeepour N, Miller PH, Pellacani D, Beer PA, Sachs K, Qiao W, Wang W, Humphries RK, Sauvageau G, Zandstra PW, Bendall SC, Nolan GP, Hansen C, Eaves CJ. Knapp DJ, et al. Blood. 2017 Jan 19;129(3):307-318. doi: 10.1182/blood-2016-09-740654. Epub 2016 Nov 8. Blood. 2017. PMID: 27827829 Free PMC article. - Differential effects of HOXB4 on nonhuman primate short- and long-term repopulating cells.
Zhang XB, Beard BC, Beebe K, Storer B, Humphries RK, Kiem HP. Zhang XB, et al. PLoS Med. 2006 May;3(5):e173. doi: 10.1371/journal.pmed.0030173. Epub 2006 May 2. PLoS Med. 2006. PMID: 16637742 Free PMC article.
References
- Ogawa M. Blood. 1993;81:2844–2853. - PubMed
- Turhan A G, Humphries R K, Phillips G L, Eaves A C, Eaves C J. N Engl J Med. 1989;320:1655–1661. - PubMed
- Cashman J, Conneally E, Petzer A, Eaves C. Exp Hematol. 1996;24:1035.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous