bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis - PubMed (original) (raw)
bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis
M E McCurrach et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Inactivation of p53-dependent apoptosis promotes oncogenic transformation, tumor development, and resistance to many cytotoxic anticancer agents. p53 can transcriptionally activate bax, a bcl-2 family member that promotes apoptosis. To determine whether bax is required for p53-dependent apoptosis, the effects of bax deficiency were examined in primary fibroblasts expressing the E1A oncogene, a setting where apoptosis is dependent on endogenous p53. We demonstrate that bax can function as an effector of p53 in chemotherapy-induced apoptosis and contributes to a p53 pathway to suppress oncogenic transformation. Furthermore, we show that additional p53 effectors participate in these processes. These p53-controlled factors act synergistically with Bax to promote a full apoptotic response, and their action is suppressed by the Bcl-2 and E1B 19K oncoproteins. These studies demonstrate that Bax is a determinant of p53-dependent chemosensitivity and illustrate how p53 can promote apoptosis by coordinating the activities of multiple effectors.
Figures
Figure 1
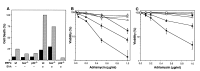
bax acts in a p53 pathway for drug-induced apoptosis. (A) Retroviruses expressing an E1A 12S cDNA and puro (LPC-12S; closed symbols) or a puro alone (LPC; open symbols) were used to infect MEFs derived from wild-type (circles), _bax_−/− (triangles), and _p53_−/− (squares) mice. Pure populations of E1A-expressing cells or controls were plated in multiwell plates and treated with the chemotherapeutic drug adriamycin. Cell viability was assessed 24 h later by trypan blue exclusion. The wild-type and _bax_−/− MEFs were from littermate embryos. Each experiment used two separate MEF preparations for each genotype, and the data were pooled. Each point represents the mean ± SD from at least three separate experiments (n ≥ 6 for each genotype). (B) Wild type (white), _bax_−/− (gray), and _p53_−/− (black) MEFs expressing E1A were treated with etoposide (2 μg/ml) or pulsed for 1 h with _cis_-platinum (100 μM), and viability was assessed after 24 h. (C) The normal and E1A-expressing cell populations described in A, together with bax+/−_p53_−/− (closed diamond) and _bax_−/−_p53_−/− (open diamond) E1A-MEFs, were treated with high doses of adriamycin to induce p53-independent apoptosis. Viability was assessed 24 h later. Each experiment used two separate MEF preparations for each genotype; the p53+/−_bax_−/− and _p53_−/−_bax_−/− E1A-MEFs were derived from littermate embryos.
Figure 2
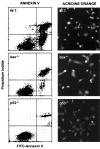
Apoptosis in _bax_-deficient E1A-MEFs. E1A-MEFs of the indicated genotype were treated with 0.1 μg/ml adriamycin for 24 h and analyzed for apoptosis by costaining with FITC-annexin V and propidium iodide, or by staining with acridine orange. Annexin V binds phosphotidylserine. Apoptotic changes in membrane biochemistry lead to increased concentration of phosphotidylserine on the outer plasma membrane, where it becomes accessible to annexin V (20). Propidium iodide fluorescently stains late apoptotic cells that have lost membrane integrity. Shown are representative dot plots from two-color flow cytometry: lower left quadrant, viable; lower right quadrant, early apoptotic; upper right, late apoptotic. Acridine orange staining allows visualization of the chromatin condensation characteristic of apoptotic cells.
Figure 3
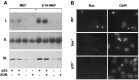
Endogenous p53 regulates bax expression in MEFs. (A) bax mRNA and protein expression in wild-type and _p53_−/− MEFs infected with either the E1A-expressing or control virus. Cell populations were treated with 0.1 μg/ml adriamycin for 9 h (ADR) or left untreated. (I) Northern blot using a murine bax cDNA probe (12), or (II) a probe corresponding to the 18S ribosomal RNA. (III) Western blot using an antipeptide rabbit polyclonal primary antibody directed against Bax. (B) Wild-type (WT), _bax_−/−, and _p53_−/− E1A-MEFs treated with 0.2 mg/ml adriamycin for 24 h. (Left) Bax expression was measured by immunofluorescence using the Bax antibody described in A. (Right) 4′, 6-diamidono-2-phenylindole (DAPI) staining of the same field reveals the condensed chromatin characteristic of apoptotic cells. The strong immunofluorescent signal observed in wild-type cells was abolished when the Bax antibody was preincubated with the synthetic peptide to which it was generated (not shown).
Figure 4
Ectopic expression of Bax, Bcl-2, and E1B 19K. (A) Primary cell populations expressing E1A and/or Bax were generated by sequential infection with recombinant retroviral vectors expressing E1A (WZL-12S) then either bax (LPC-HAbax; gray bars) or a empty vector (LPC; black bars). Infected populations were plated in multiwell plates and analyzed for viability 24 h later without drug treatment. The MEF genotypes and E1A status are indicated below the graph. Shown is a representative graph of three separate experiments that all produced the same results. (B) Wild-type (circles), _bax_−/− (triangles), or _p53_−/− (squares) MEFs were first infected with retroviruses expressing E1A (WZL-12S) and then either Bcl-2 (LPC-bcl2; open symbols) or a control vector (LPC; closed symbols). After selection, cells were treated with adriamycin, and viability was determined after 24 h. (C) E1A-expressing populations were superinfected with a retrovirus expressing E1B 19K (LPC-19K) and analyzed as in B. The symbols are as described in B, except that open symbols represent cells coexpressing E1B 19K with E1A. Each point in B and C represents the average ± SD of data obtained from at least three separate experiments.
Figure 5
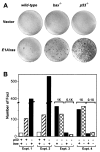
bax acts in a p53 pathway to suppress oncogenic transformation. Wild-type (p53+/+bax+/+), bax null (p53+/+_bax_−/−), p53 null (p53_−/−_bax+/+) and double mutant (_p53_−/−_bax_−/−) MEFs were transfected with plasmids expressing adenovirus-5 E1A and an activated ras oncogene. Two weeks after transfection, plates were fixed and stained with Giemsa to visualize foci. (A) Photographs of representative plates. (B) Graph quantifying the number of foci from several experiments. The genotypes are indicated below the graph and are as follows: for p53, (+) indicates p53+/+ and (−) indicates _p53_−/−; for bax, (+) indicates bax+/+ and (−) indicates _bax_−/−. In Exp. 1 and 2, the number of foci on p53_−/−_bax+/+ were such that the numbers must be considered estimates. Exp. 3 and 4, two different amounts of input E1A/ras DNA were used to generate numbers of foci that could be accurately counted. The amount of E1A/ras DNA in the 0.1× lanes was 1/10 those marked 1×.
Similar articles
- The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein.
Han J, Sabbatini P, Perez D, Rao L, Modha D, White E. Han J, et al. Genes Dev. 1996 Feb 15;10(4):461-77. doi: 10.1101/gad.10.4.461. Genes Dev. 1996. PMID: 8600029 - Bax accelerates tumorigenesis in p53-deficient mice.
Knudson CM, Johnson GM, Lin Y, Korsmeyer SJ. Knudson CM, et al. Cancer Res. 2001 Jan 15;61(2):659-65. Cancer Res. 2001. PMID: 11212265 - Transcriptional inactivation of p53, Bax, Bcl-2 and Mdm2 correlates with malignant transformation of the uterine cervix.
Soufla G, Baritaki S, Sifakis S, Zafiropoulos A, Spandidos DA. Soufla G, et al. Int J Biol Markers. 2005 Jan-Mar;20(1):18-27. Int J Biol Markers. 2005. PMID: 15832769 - P53 null mice: damaging the hypothesis?
Sansom OJ, Clarke AR. Sansom OJ, et al. Mutat Res. 2000 Sep 18;452(2):149-62. doi: 10.1016/s0027-5107(00)00089-0. Mutat Res. 2000. PMID: 11024475 Review. - Bcl-2 family proteins: regulators of chemoresistance in cancer.
Reed JC. Reed JC. Toxicol Lett. 1995 Dec;82-83:155-8. doi: 10.1016/0378-4274(95)03551-6. Toxicol Lett. 1995. PMID: 8597045 Review.
Cited by
- Cancer Metabolism and the Evasion of Apoptotic Cell Death.
Sharma A, Boise LH, Shanmugam M. Sharma A, et al. Cancers (Basel). 2019 Aug 9;11(8):1144. doi: 10.3390/cancers11081144. Cancers (Basel). 2019. PMID: 31405035 Free PMC article. Review. - Simian virus 40 large T antigen and two independent T-antigen segments sensitize cells to apoptosis following genotoxic damage.
Cole SL, Tevethia MJ. Cole SL, et al. J Virol. 2002 Aug;76(16):8420-32. doi: 10.1128/jvi.76.16.8420-8432.2002. J Virol. 2002. PMID: 12134045 Free PMC article. - Microsatellite instability and frameshift mutations in the Bax gene in hereditary nonpolyposis colorectal carcinoma.
Sakao Y, Noro M, Sekine S, Nozue M, Hirohashi S, Itoh T, Noguchi M. Sakao Y, et al. Jpn J Cancer Res. 1998 Oct;89(10):1020-7. doi: 10.1111/j.1349-7006.1998.tb00491.x. Jpn J Cancer Res. 1998. PMID: 9849580 Free PMC article. - Genetic and functional characterization of disease associations explains comorbidity.
Rubio-Perez C, Guney E, Aguilar D, Piñero J, Garcia-Garcia J, Iadarola B, Sanz F, Fernandez-Fuentes N, Furlong LI, Oliva B. Rubio-Perez C, et al. Sci Rep. 2017 Jul 24;7(1):6207. doi: 10.1038/s41598-017-04939-4. Sci Rep. 2017. PMID: 28740175 Free PMC article. - Regulation of Mdm2-directed degradation by the C terminus of p53.
Kubbutat MH, Ludwig RL, Ashcroft M, Vousden KH. Kubbutat MH, et al. Mol Cell Biol. 1998 Oct;18(10):5690-8. doi: 10.1128/MCB.18.10.5690. Mol Cell Biol. 1998. PMID: 9742086 Free PMC article.
References
- Elrouby S, Thomas A, Costin D, Rosenberg C R, Potmesil M, Silber R, Newcomb E W. Blood. 1993;82:3452–3459. - PubMed
- Wattel E, Preudhomme C, Hecquet B, Vanrumbeke M, Quesnel B, Dervite I, Morel P, Fenaux P. Blood. 1994;84:3148–3157. - PubMed
- Bergh J, Norberg T, Sjogren S, Lindgren A, Holmberg L. Nat Med. 1995;1:1029–1034. - PubMed
- Aas T, Borresen A, Geisler S, Smith-Sorrensen B, Johnsen H, Varhaug J E, Akslen L A, Lonning P E. Nat Med. 1996;2:811–814. - PubMed
- Righetti S C, Della Torre G, Pilotti S, Menard S, Ottone F, Colnaghi M I, Pierotti M A, Lavarino C, Cornarotti M, Oriana S, Bohm S, Bresciani G L, Spatti G, Zunino F. Cancer Res. 1996;56:689–693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous