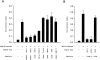ICAMs redistributed by chemokines to cellular uropods as a mechanism for recruitment of T lymphocytes - PubMed (original) (raw)
ICAMs redistributed by chemokines to cellular uropods as a mechanism for recruitment of T lymphocytes
M A del Pozo et al. J Cell Biol. 1997.
Abstract
The recruitment of leukocytes from the bloodstream is a key step in the inflammatory reaction, and chemokines are among the main regulators of this process. During lymphocyte-endothelial interaction, chemokines induce the polarization of T lymphocytes, with the formation of a cytoplasmic projection (uropod) and redistribution of several adhesion molecules (ICAM-1,-3, CD43, CD44) to this structure. Although it has been reported that these cytokines regulate the adhesive state of integrins in leukocytes, their precise mechanisms of chemoattraction remain to be elucidated. We have herein studied the functional role of the lymphocyte uropod. Confocal microscopy studies clearly showed that cell uropods project away from the cell bodies of adhered lymphocytes and that polarized T cells contact other T cells through the uropod structure. Time-lapse videomicroscopy studies revealed that uropod-bearing T cells were able, through this cellular projection, to contact, capture, and transport additional bystander T cells. Quantitative analysis revealed that the induction of uropods results in a 5-10-fold increase in cell recruitment. Uropod-mediated cell recruitment seems to have physiological relevance, since it was promoted by both CD45R0+ peripheral blood memory T cells as well as by in vivo activated lymphocytes. Additional studies showed that the cell recruitment mediated by uropods was abrogated with antibodies to ICAM-1, -3, and LFA-1, whereas mAb to CD43, CD44, CD45, and L-selectin did not have a significant effect, thus indicating that the interaction of LFA-1 with ICAM-1 and -3 appears to be responsible for this process. To determine whether the increment in cell recruitment mediated by uropod may affect the transendothelial migration of T cells, we carried out chemotaxis assays through confluent monolayers of endothelial cells specialized in lymphocyte extravasation. An enhancement of T cell migration was observed under conditions of uropod formation, and this increase was prevented by incubation with either blocking anti-ICAM-3 mAbs or drugs that impair uropod formation. These data indicate that the cell interactions mediated by cell uropods represent a cooperative mechanism in lymphocyte recruitment, which may act as an amplification system in the inflammatory response.
Figures
Figure 1
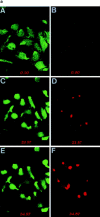
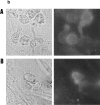
Cell uropods emerge away from the flattened cell bodies of adhered lymphocytes and are projected to the outer milieu. (a) Confocal fluorescence microscopy analysis of the spatial orientation of the uropod and ICAM-3 localization on T lymphoblasts adhering to ICAM-1–coated surfaces. T cells labeled with the fluorescence cytoplasmic probe CFDA-SE (green fluorescence) were allowed to bind to coverslips coated with 10 μg/ml of ICAM-1–Fc for 30 min at 37°C in the presence of the uropod inducing anti–ICAM-3 HP2/19 mAb (5 μg/ml). Cells were then fixed and stained for ICAM-3 (red fluorescence) as described in Materials and Methods. Slides were analyzed by confocal laser scanning microscopy, and optical sectioning was adjusted to the plane of adhesion (A and B), 7 (C and D), and 10 μm above (E and F). Optical sections correspond to 0.3 μm in thickness. (b) T lymphoblasts were allowed to adhere to ICAM-1 in the presence (right column) or in the absence (left column) of 10 ng/ml RANTES, and samples were processed as in a. Cytoplasmic (green fluorescence) and ICAM-3 membrane staining (red fluorescence) are shown. Optical sectioning was adjusted to the plane of adhesion (A), 5 (B), and 8 μm above that plane (C).
Figure 1
Cell uropods emerge away from the flattened cell bodies of adhered lymphocytes and are projected to the outer milieu. (a) Confocal fluorescence microscopy analysis of the spatial orientation of the uropod and ICAM-3 localization on T lymphoblasts adhering to ICAM-1–coated surfaces. T cells labeled with the fluorescence cytoplasmic probe CFDA-SE (green fluorescence) were allowed to bind to coverslips coated with 10 μg/ml of ICAM-1–Fc for 30 min at 37°C in the presence of the uropod inducing anti–ICAM-3 HP2/19 mAb (5 μg/ml). Cells were then fixed and stained for ICAM-3 (red fluorescence) as described in Materials and Methods. Slides were analyzed by confocal laser scanning microscopy, and optical sectioning was adjusted to the plane of adhesion (A and B), 7 (C and D), and 10 μm above (E and F). Optical sections correspond to 0.3 μm in thickness. (b) T lymphoblasts were allowed to adhere to ICAM-1 in the presence (right column) or in the absence (left column) of 10 ng/ml RANTES, and samples were processed as in a. Cytoplasmic (green fluorescence) and ICAM-3 membrane staining (red fluorescence) are shown. Optical sectioning was adjusted to the plane of adhesion (A), 5 (B), and 8 μm above that plane (C).
Figure 2
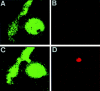
Lymphocyte uropods promote cell contacts between T lymphoblasts. Confocal microscopy analysis of lymphocyte–lymphocyte interactions mediated by cell uropods. T lymphoblasts labeled with the green fluorescent cytoplasmic probe CFDA-SE were allowed to bind to ICAM-1–Fc–coated coverslips (10 μg/ml) for 30 min at 37°C in the presence of the uropod inducing anti–ICAM-3 HP2/19 mAb. Cells were then fixed and stained for ICAM-3 (red fluorescence). Slides were analyzed as described in Materials and Methods. Optical sections were adjusted to the plane of adhesion (A and B) or 5 μm above (C and D). E corresponds to the three-dimensional reconstruction of cells, simultaneously showing the cytoplasmic (green) and ICAM-3 (red) staining.
Figure 2
Lymphocyte uropods promote cell contacts between T lymphoblasts. Confocal microscopy analysis of lymphocyte–lymphocyte interactions mediated by cell uropods. T lymphoblasts labeled with the green fluorescent cytoplasmic probe CFDA-SE were allowed to bind to ICAM-1–Fc–coated coverslips (10 μg/ml) for 30 min at 37°C in the presence of the uropod inducing anti–ICAM-3 HP2/19 mAb. Cells were then fixed and stained for ICAM-3 (red fluorescence). Slides were analyzed as described in Materials and Methods. Optical sections were adjusted to the plane of adhesion (A and B) or 5 μm above (C and D). E corresponds to the three-dimensional reconstruction of cells, simultaneously showing the cytoplasmic (green) and ICAM-3 (red) staining.
Figure 3
T cells are recruited by uropod-bearing T lymphocytes. Time-lapse videomicroscopy analysis of lymphocyte–lymphocyte interactions. (A and B) The chemokine RANTES and the uropod-inducing anti–ICAM-3 HP2/19 mAb induce T cells to contact, capture, and transport other T cells through their uropods. T lymphoblasts were allowed to bind to plastic petri dishes coated with ICAM-1–Fc and treated with the anti–ICAM-3 uropod-inducing mAb HP2/19 (Ab, 5 μg/ml), with the isotype-matched nonuropod inducing anti– ICAM-3 TP1/24 (Aa, 5 μg/ml), or with 10 ng/ml RANTES (B) for 30 min at 37°C. After addition of a second cohort of untreated T lymphocytes (exhibiting a phase-bright appearance readily distinguishable from the phase-dark cells of the first cohort), cell–cell interactions were filmed with a time-lapse videocassette recorder for 1 h. Time frames obtained from videotape recording of one representative experiment are shown. White arrowheads point to uropods displayed by phase-dark cells of the first layer; black arrows indicate phasebright T lymphocytes captured by uropod-bearing cells, while the small white arrows mark the direction of movement of the adhered cell. (Inset) Polarized lymphocyte migrating on ICAM-1–coated surface, showing the phase-dark leading edge (L) and the phase-bright uropod (U). (C) Measurement of cell recruitment mediated by cell uropods. T lymphoblasts were allowed to bind to plastic petri dishes coated with VCAM-1–Fc for 30 min at 37°C in the presence of medium alone, 10 ng/ml RANTES, MCP-1, MIP-1α, MIP-1β, IL-8, 20 ng/ ml PMA, or 5 μg/ml anti–ICAM-3 HP2/19 and TP1/24 mAb. After addition of a second cohort of T cells, cell–cell interactions were recorded for 1 h, and the recruitment index was estimated as described in Materials and Methods. Arithmetic mean ± 1 SD of three independent experiments is shown.
Figure 3
T cells are recruited by uropod-bearing T lymphocytes. Time-lapse videomicroscopy analysis of lymphocyte–lymphocyte interactions. (A and B) The chemokine RANTES and the uropod-inducing anti–ICAM-3 HP2/19 mAb induce T cells to contact, capture, and transport other T cells through their uropods. T lymphoblasts were allowed to bind to plastic petri dishes coated with ICAM-1–Fc and treated with the anti–ICAM-3 uropod-inducing mAb HP2/19 (Ab, 5 μg/ml), with the isotype-matched nonuropod inducing anti– ICAM-3 TP1/24 (Aa, 5 μg/ml), or with 10 ng/ml RANTES (B) for 30 min at 37°C. After addition of a second cohort of untreated T lymphocytes (exhibiting a phase-bright appearance readily distinguishable from the phase-dark cells of the first cohort), cell–cell interactions were filmed with a time-lapse videocassette recorder for 1 h. Time frames obtained from videotape recording of one representative experiment are shown. White arrowheads point to uropods displayed by phase-dark cells of the first layer; black arrows indicate phasebright T lymphocytes captured by uropod-bearing cells, while the small white arrows mark the direction of movement of the adhered cell. (Inset) Polarized lymphocyte migrating on ICAM-1–coated surface, showing the phase-dark leading edge (L) and the phase-bright uropod (U). (C) Measurement of cell recruitment mediated by cell uropods. T lymphoblasts were allowed to bind to plastic petri dishes coated with VCAM-1–Fc for 30 min at 37°C in the presence of medium alone, 10 ng/ml RANTES, MCP-1, MIP-1α, MIP-1β, IL-8, 20 ng/ ml PMA, or 5 μg/ml anti–ICAM-3 HP2/19 and TP1/24 mAb. After addition of a second cohort of T cells, cell–cell interactions were recorded for 1 h, and the recruitment index was estimated as described in Materials and Methods. Arithmetic mean ± 1 SD of three independent experiments is shown.
Figure 3
T cells are recruited by uropod-bearing T lymphocytes. Time-lapse videomicroscopy analysis of lymphocyte–lymphocyte interactions. (A and B) The chemokine RANTES and the uropod-inducing anti–ICAM-3 HP2/19 mAb induce T cells to contact, capture, and transport other T cells through their uropods. T lymphoblasts were allowed to bind to plastic petri dishes coated with ICAM-1–Fc and treated with the anti–ICAM-3 uropod-inducing mAb HP2/19 (Ab, 5 μg/ml), with the isotype-matched nonuropod inducing anti– ICAM-3 TP1/24 (Aa, 5 μg/ml), or with 10 ng/ml RANTES (B) for 30 min at 37°C. After addition of a second cohort of untreated T lymphocytes (exhibiting a phase-bright appearance readily distinguishable from the phase-dark cells of the first cohort), cell–cell interactions were filmed with a time-lapse videocassette recorder for 1 h. Time frames obtained from videotape recording of one representative experiment are shown. White arrowheads point to uropods displayed by phase-dark cells of the first layer; black arrows indicate phasebright T lymphocytes captured by uropod-bearing cells, while the small white arrows mark the direction of movement of the adhered cell. (Inset) Polarized lymphocyte migrating on ICAM-1–coated surface, showing the phase-dark leading edge (L) and the phase-bright uropod (U). (C) Measurement of cell recruitment mediated by cell uropods. T lymphoblasts were allowed to bind to plastic petri dishes coated with VCAM-1–Fc for 30 min at 37°C in the presence of medium alone, 10 ng/ml RANTES, MCP-1, MIP-1α, MIP-1β, IL-8, 20 ng/ ml PMA, or 5 μg/ml anti–ICAM-3 HP2/19 and TP1/24 mAb. After addition of a second cohort of T cells, cell–cell interactions were recorded for 1 h, and the recruitment index was estimated as described in Materials and Methods. Arithmetic mean ± 1 SD of three independent experiments is shown.
Figure 4
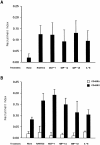
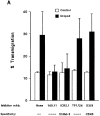
Freshly isolated CD45RO+ lymphocytes are able to recruit cells through the uropod. (A and B) Measurement of cell recruitment mediated by cell uropods. The total population of PBL (A), CD45RA+, or CD45RO+ cells (B) were allowed to bind to plastic petri dishes coated with ICAM-1–Fc for 30 min at 37°C in the presence of medium alone, 10 ng/ml RANTES, MCP-1, MIP1α, MIP-1β, or IL-8. After addition of a second cohort of PBL from the same donor, cell–cell interactions were recorded for 1 h, and the recruitment index was estimated as described in Materials and Methods. Arithmetic mean ± 1 SD of four independent experiments performed with PBL (A) or two independent ones with CD45RA+/CD45RO+ cells (B) is shown. (C) Freshly isolated CD45RA+ (a and c) or CD45RO+ (b and d) cells were allowed to bind to coverslips coated with 10 μg/ml ICAM-1–Fc for 30 min at 37°C in the presence (c and d) or in the absence (a and b) of 10 ng/ml MIP-1α. Fixed cells were then stained for ICAM-3 with TP1/24 mAb, and the proportion of uropod-bearing cells was calculated as described in Materials and Methods. The percentage of polarized cells in CD45RA+ versus CD45RO+ cells was as follows: untreated 5 vs 19%, RANTES 11 vs 29%, MCP-1 10 vs 32%, MIP-1α 3 vs 26%, MIP-1β 4 vs 22%, and IL-8 8 vs 20%. The cellular uropod and the cell contact area with the substratum were indeed in a distinct plane of focus.
Figure 4
Freshly isolated CD45RO+ lymphocytes are able to recruit cells through the uropod. (A and B) Measurement of cell recruitment mediated by cell uropods. The total population of PBL (A), CD45RA+, or CD45RO+ cells (B) were allowed to bind to plastic petri dishes coated with ICAM-1–Fc for 30 min at 37°C in the presence of medium alone, 10 ng/ml RANTES, MCP-1, MIP1α, MIP-1β, or IL-8. After addition of a second cohort of PBL from the same donor, cell–cell interactions were recorded for 1 h, and the recruitment index was estimated as described in Materials and Methods. Arithmetic mean ± 1 SD of four independent experiments performed with PBL (A) or two independent ones with CD45RA+/CD45RO+ cells (B) is shown. (C) Freshly isolated CD45RA+ (a and c) or CD45RO+ (b and d) cells were allowed to bind to coverslips coated with 10 μg/ml ICAM-1–Fc for 30 min at 37°C in the presence (c and d) or in the absence (a and b) of 10 ng/ml MIP-1α. Fixed cells were then stained for ICAM-3 with TP1/24 mAb, and the proportion of uropod-bearing cells was calculated as described in Materials and Methods. The percentage of polarized cells in CD45RA+ versus CD45RO+ cells was as follows: untreated 5 vs 19%, RANTES 11 vs 29%, MCP-1 10 vs 32%, MIP-1α 3 vs 26%, MIP-1β 4 vs 22%, and IL-8 8 vs 20%. The cellular uropod and the cell contact area with the substratum were indeed in a distinct plane of focus.
Figure 5
Lymphocyte polarization occurs in vivo. (a) Lymphocyte polarization and ICAM-3 redistribution to the cellular uropod during TIL binding to autologous melanoma tumor cells. TIL were cocultured with a monolayer of melanoma cells from the same patient for 1 h at 37°C. Fixed cells were then stained for ICAM-3 with TP1/24 mAb followed by incubation with a Cy3-goat anti–mouse IgG, as described in Materials and Methods. Epifluorescent and bright field conditions were photographed on the same frame by double exposure. Arrows point to uropods. (b) Tissue distribution of ICAM-3 in T lymphocytes that infiltrate a tumor specimen. A tissue section of a lung metastatic melanoma biopsy was double immunofluorescence stained for ICAM-3 (red fluorescence) and CD3 (green fluorescence) as described in Materials and Methods. Tissue sections were analyzed by confocal laser scanning microscopy. Serial optical sections of 0.5 μm thick are shown (from A to E). A small aggregate of three lymphocytes connected through the tissue is followed. Arrows point out the area of cell–cell contact where ICAM-3 is concentrated.
Figure 5
Lymphocyte polarization occurs in vivo. (a) Lymphocyte polarization and ICAM-3 redistribution to the cellular uropod during TIL binding to autologous melanoma tumor cells. TIL were cocultured with a monolayer of melanoma cells from the same patient for 1 h at 37°C. Fixed cells were then stained for ICAM-3 with TP1/24 mAb followed by incubation with a Cy3-goat anti–mouse IgG, as described in Materials and Methods. Epifluorescent and bright field conditions were photographed on the same frame by double exposure. Arrows point to uropods. (b) Tissue distribution of ICAM-3 in T lymphocytes that infiltrate a tumor specimen. A tissue section of a lung metastatic melanoma biopsy was double immunofluorescence stained for ICAM-3 (red fluorescence) and CD3 (green fluorescence) as described in Materials and Methods. Tissue sections were analyzed by confocal laser scanning microscopy. Serial optical sections of 0.5 μm thick are shown (from A to E). A small aggregate of three lymphocytes connected through the tissue is followed. Arrows point out the area of cell–cell contact where ICAM-3 is concentrated.
Figure 6
TIL are able to recruit cells through the uropod. (a) PBL or TIL were allowed to bind to plastic petri dishes coated with 10 μg/ml ICAM-1–Fc or 20 μg/ml FN80 for 30 min at 37°C. After addition of a second cohort of PBL, cell–cell interactions were recorded for 1 h, and the recruitment index was estimated as described in Materials and Methods. Arithmetic mean ± 1 SD of three independent experiments performed with PBL and TIL from three different donors is shown. In parallel, lymphocytes were allowed to adhere to coverslips coated with 10 μg/ml ICAM-1–Fc for 30 min at 37°C, fixed, and stained for ICAM-3, and the proportion of uropod-bearing cells was calculated as described in Materials and Methods: on ICAM-1, PBL 3%, TIL 17%; on FN80, PBL 5%, TIL 23%. (b) TIL were allowed to bind to plastic petri dishes coated with a monolayer of melanoma cells from the same patient for 1 h at 37°C. A second cohort of PBL was added, and after 1 h of incubation the specimens were fixed and stained for ICAM-3 with TP1/24 mAb as described in Materials and Methods. Same fields from two different specimens (A and B) were photographed under bright field (left column) and epifluorescent (right column) conditions.
Figure 6
TIL are able to recruit cells through the uropod. (a) PBL or TIL were allowed to bind to plastic petri dishes coated with 10 μg/ml ICAM-1–Fc or 20 μg/ml FN80 for 30 min at 37°C. After addition of a second cohort of PBL, cell–cell interactions were recorded for 1 h, and the recruitment index was estimated as described in Materials and Methods. Arithmetic mean ± 1 SD of three independent experiments performed with PBL and TIL from three different donors is shown. In parallel, lymphocytes were allowed to adhere to coverslips coated with 10 μg/ml ICAM-1–Fc for 30 min at 37°C, fixed, and stained for ICAM-3, and the proportion of uropod-bearing cells was calculated as described in Materials and Methods: on ICAM-1, PBL 3%, TIL 17%; on FN80, PBL 5%, TIL 23%. (b) TIL were allowed to bind to plastic petri dishes coated with a monolayer of melanoma cells from the same patient for 1 h at 37°C. A second cohort of PBL was added, and after 1 h of incubation the specimens were fixed and stained for ICAM-3 with TP1/24 mAb as described in Materials and Methods. Same fields from two different specimens (A and B) were photographed under bright field (left column) and epifluorescent (right column) conditions.
Figure 7
Involvement of ICAM-1 and -3 in lymphocyte recruitment mediated by cell uropods. (A) T lymphoblasts adhered for 30 min at 37°C to VCAM-1–Fc coated surfaces in the presence of 10 ng/ml RANTES were then incubated for 15 min with the blocking mAb anti–ICAM-3 140.11, anti–ICAM-1 Hu5/3, anti-CD43 TP1/36, antiCD44 HP2/9, or anti-CD45 D3/9 before the addition of a second cohort of T cells. Where indicated, the latter cells were pretreated for 15 min with the anti-β2 integrin Lia3/2 mAb, the anti-CD11a YTH81.5 mAb, or the anti– L-selectin LAM1-3 mAb before addition to the first layer of T cells. The recruitment index was calculated. Arithmetic mean ± 1 SD of three independent experiments is shown. (B) Cells were allowed to adhere for 30 min at 37°C to ICAM-1–Fc coated petri dishes in the presence of the anti–ICAM-3 uropod-inducing mAb HP2/19 and additionally incubated for 15 min at 37°C either with the blocking anti–ICAM-3 mAbs 140.11 or ICR2.1 or with the anti-CD45 D3/9 mAb before addition of the second cohort of T cells. Arithmetic mean ± 1 SD of two independent experiments is shown.
Figure 8
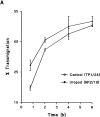
Effect of uropod induction on transendothelial migration of T lymphocytes. (A and B) Uropod induction increases transendothelial migration. Migration of T lymphoblasts across confluent monolayers from a human dermal microvascular endothelial cell line (HMEC-1) was assayed in a Transwell cell culture chamber. T lymphoblasts were allowed to bind to confluent EC monolayers cultured on polycarbonate membranes and treated either with the uropod-inducing mAb HP2/19 or with the noninducing anti–ICAM-3 mAb TP1/24. A second cohort of 106 51Cr-labeled T cells was then added to the upper compartment of the chamber and incubated for long (A, from 45 min to 6 h) and short (B, from 5 to 60 min) periods of time at 37°C in a 5% CO2 atmosphere. The percentage of cells that migrated to the lower well of the chamber was calculated using a γ counter. A representative experiment out of five independent ones run in quadruplicate is shown. Error bars represent ± 1 SD of values from quadruplicate Transwell chambers. (C) The increment in migration was dependent on the number of cells in the first layer of lymphocytes. In similar experiments of transendothelial migration, different numbers of T lymphoblasts were incubated with the confluent EC monolayers, and migration of a second cohort of 51Cr-labeled T cells was analyzed. Transmigration is presented as the ratio between percentage of migrated cells under uropod conditions and percentage of migrated cells under nonuropod conditions. Arithmethic mean ± 1 SD of three independent experiments is shown.
Figure 8
Effect of uropod induction on transendothelial migration of T lymphocytes. (A and B) Uropod induction increases transendothelial migration. Migration of T lymphoblasts across confluent monolayers from a human dermal microvascular endothelial cell line (HMEC-1) was assayed in a Transwell cell culture chamber. T lymphoblasts were allowed to bind to confluent EC monolayers cultured on polycarbonate membranes and treated either with the uropod-inducing mAb HP2/19 or with the noninducing anti–ICAM-3 mAb TP1/24. A second cohort of 106 51Cr-labeled T cells was then added to the upper compartment of the chamber and incubated for long (A, from 45 min to 6 h) and short (B, from 5 to 60 min) periods of time at 37°C in a 5% CO2 atmosphere. The percentage of cells that migrated to the lower well of the chamber was calculated using a γ counter. A representative experiment out of five independent ones run in quadruplicate is shown. Error bars represent ± 1 SD of values from quadruplicate Transwell chambers. (C) The increment in migration was dependent on the number of cells in the first layer of lymphocytes. In similar experiments of transendothelial migration, different numbers of T lymphoblasts were incubated with the confluent EC monolayers, and migration of a second cohort of 51Cr-labeled T cells was analyzed. Transmigration is presented as the ratio between percentage of migrated cells under uropod conditions and percentage of migrated cells under nonuropod conditions. Arithmethic mean ± 1 SD of three independent experiments is shown.
Figure 8
Effect of uropod induction on transendothelial migration of T lymphocytes. (A and B) Uropod induction increases transendothelial migration. Migration of T lymphoblasts across confluent monolayers from a human dermal microvascular endothelial cell line (HMEC-1) was assayed in a Transwell cell culture chamber. T lymphoblasts were allowed to bind to confluent EC monolayers cultured on polycarbonate membranes and treated either with the uropod-inducing mAb HP2/19 or with the noninducing anti–ICAM-3 mAb TP1/24. A second cohort of 106 51Cr-labeled T cells was then added to the upper compartment of the chamber and incubated for long (A, from 45 min to 6 h) and short (B, from 5 to 60 min) periods of time at 37°C in a 5% CO2 atmosphere. The percentage of cells that migrated to the lower well of the chamber was calculated using a γ counter. A representative experiment out of five independent ones run in quadruplicate is shown. Error bars represent ± 1 SD of values from quadruplicate Transwell chambers. (C) The increment in migration was dependent on the number of cells in the first layer of lymphocytes. In similar experiments of transendothelial migration, different numbers of T lymphoblasts were incubated with the confluent EC monolayers, and migration of a second cohort of 51Cr-labeled T cells was analyzed. Transmigration is presented as the ratio between percentage of migrated cells under uropod conditions and percentage of migrated cells under nonuropod conditions. Arithmethic mean ± 1 SD of three independent experiments is shown.
Figure 9
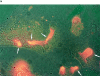
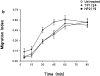
Uropod induction increases migration across HEC. (a) Interaction through a uropod-like structure between migrated T cells and bound lymphocytes. D shows the usual morphology of high endothelial vein cells. A–C show the same field photographed in a different plane of focus, from the level of HEC (A) to the level of surface bound lymphocytes (C). E shows a different field. White arrows point to cell–cell contacts through a uropodlike structure. (b) T lymphoblasts preincubated with the indicated mAb were added to confluent cultures of HEC in multichamber polystyrene slides and incubated for different periods of time. After washing, adherent lymphocytes were identified as either “surface bound” or “transmigrated” by counterstaining with toluidine blue and high power light microscopy; the migration index was calculated as stated in Materials and Methods. A representative experiment out of three independent ones run in triplicate is shown. (c) In parallel, some specimens were stained for ICAM-3 after fixation as described in Materials and Methods. Same fields were photographed under bright field (A) and epifluorescent (B) conditions. Arrows point to ICAM-3+ uropods.
Figure 9
Uropod induction increases migration across HEC. (a) Interaction through a uropod-like structure between migrated T cells and bound lymphocytes. D shows the usual morphology of high endothelial vein cells. A–C show the same field photographed in a different plane of focus, from the level of HEC (A) to the level of surface bound lymphocytes (C). E shows a different field. White arrows point to cell–cell contacts through a uropodlike structure. (b) T lymphoblasts preincubated with the indicated mAb were added to confluent cultures of HEC in multichamber polystyrene slides and incubated for different periods of time. After washing, adherent lymphocytes were identified as either “surface bound” or “transmigrated” by counterstaining with toluidine blue and high power light microscopy; the migration index was calculated as stated in Materials and Methods. A representative experiment out of three independent ones run in triplicate is shown. (c) In parallel, some specimens were stained for ICAM-3 after fixation as described in Materials and Methods. Same fields were photographed under bright field (A) and epifluorescent (B) conditions. Arrows point to ICAM-3+ uropods.
Figure 9
Uropod induction increases migration across HEC. (a) Interaction through a uropod-like structure between migrated T cells and bound lymphocytes. D shows the usual morphology of high endothelial vein cells. A–C show the same field photographed in a different plane of focus, from the level of HEC (A) to the level of surface bound lymphocytes (C). E shows a different field. White arrows point to cell–cell contacts through a uropodlike structure. (b) T lymphoblasts preincubated with the indicated mAb were added to confluent cultures of HEC in multichamber polystyrene slides and incubated for different periods of time. After washing, adherent lymphocytes were identified as either “surface bound” or “transmigrated” by counterstaining with toluidine blue and high power light microscopy; the migration index was calculated as stated in Materials and Methods. A representative experiment out of three independent ones run in triplicate is shown. (c) In parallel, some specimens were stained for ICAM-3 after fixation as described in Materials and Methods. Same fields were photographed under bright field (A) and epifluorescent (B) conditions. Arrows point to ICAM-3+ uropods.
Figure 10
T cell migration mediated by cell uropods is prevented by blocking with anti–ICAM-3 mAbs or with drugs that impair uropod formation. T cell migration across HMEC-1 was assayed as described in Materials and Methods. (A) After treatment of the first layer of T lymphoblasts with either HP2/19 (uropod) or TP1/ 24 (control) anti–ICAM-3 mAbs, cells were incubated for additional 15 min with the indicated blocking mAbs. (B) Before the incubation of the first layer of T cells with the EC monolayers, lymphocytes were treated either with butanedione monoxime or with colchicine for 30 min at 37°C, and then the drugs were extensively washed. In parallel, drug-treated lymphocytes were allowed to adhere to coverslips coated with 10 μg/ml ICAM-1–Fc for 30 min at 37°C, fixed and stained for ICAM-3, and the proportion of uropod-bearing cells was calculated as described in Materials and Methods: untreated control, 11%, uropod, 76%; butanedione monoxime control, 1%, uropod 4%; colchicine control, 40%, uropod, 81%. A representative experiment out of three independent ones run in duplicate is shown.
Figure 10
T cell migration mediated by cell uropods is prevented by blocking with anti–ICAM-3 mAbs or with drugs that impair uropod formation. T cell migration across HMEC-1 was assayed as described in Materials and Methods. (A) After treatment of the first layer of T lymphoblasts with either HP2/19 (uropod) or TP1/ 24 (control) anti–ICAM-3 mAbs, cells were incubated for additional 15 min with the indicated blocking mAbs. (B) Before the incubation of the first layer of T cells with the EC monolayers, lymphocytes were treated either with butanedione monoxime or with colchicine for 30 min at 37°C, and then the drugs were extensively washed. In parallel, drug-treated lymphocytes were allowed to adhere to coverslips coated with 10 μg/ml ICAM-1–Fc for 30 min at 37°C, fixed and stained for ICAM-3, and the proportion of uropod-bearing cells was calculated as described in Materials and Methods: untreated control, 11%, uropod, 76%; butanedione monoxime control, 1%, uropod 4%; colchicine control, 40%, uropod, 81%. A representative experiment out of three independent ones run in duplicate is shown.
Similar articles
- Chemokines regulate cellular polarization and adhesion receptor redistribution during lymphocyte interaction with endothelium and extracellular matrix. Involvement of cAMP signaling pathway.
del Pozo MA, Sánchez-Mateos P, Nieto M, Sánchez-Madrid F. del Pozo MA, et al. J Cell Biol. 1995 Oct;131(2):495-508. doi: 10.1083/jcb.131.2.495. J Cell Biol. 1995. PMID: 7593174 Free PMC article. - Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization.
Serrador JM, Alonso-Lebrero JL, del Pozo MA, Furthmayr H, Schwartz-Albiez R, Calvo J, Lozano F, Sánchez-Madrid F. Serrador JM, et al. J Cell Biol. 1997 Sep 22;138(6):1409-23. doi: 10.1083/jcb.138.6.1409. J Cell Biol. 1997. PMID: 9298994 Free PMC article. - CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts.
Serrador JM, Nieto M, Alonso-Lebrero JL, del Pozo MA, Calvo J, Furthmayr H, Schwartz-Albiez R, Lozano F, González-Amaro R, Sánchez-Mateos P, Sánchez-Madrid F. Serrador JM, et al. Blood. 1998 Jun 15;91(12):4632-44. Blood. 1998. PMID: 9616160 - The two poles of the lymphocyte: specialized cell compartments for migration and recruitment.
del Pozo MA, Nieto M, Serrador JM, Sancho D, Vicente-Manzanares M, Martínez C, Sánchez-Madrid F. del Pozo MA, et al. Cell Adhes Commun. 1998;6(2-3):125-33. doi: 10.3109/15419069809004468. Cell Adhes Commun. 1998. PMID: 9823463 Review. - Molecular mechanisms involved in T cell migration across the blood-brain barrier.
Engelhardt B. Engelhardt B. J Neural Transm (Vienna). 2006 Apr;113(4):477-85. doi: 10.1007/s00702-005-0409-y. J Neural Transm (Vienna). 2006. PMID: 16550326 Review.
Cited by
- Paracrine interactions during human implantation.
Domínguez F, Remohí J, Pellicer A, Simón C. Domínguez F, et al. Rev Endocr Metab Disord. 2002 May;3(2):97-105. doi: 10.1023/a:1015498610742. Rev Endocr Metab Disord. 2002. PMID: 12007286 Review. No abstract available. - Bringing up the rear: defining the roles of the uropod.
Sánchez-Madrid F, Serrador JM. Sánchez-Madrid F, et al. Nat Rev Mol Cell Biol. 2009 May;10(5):353-9. doi: 10.1038/nrm2680. Epub 2009 Apr 17. Nat Rev Mol Cell Biol. 2009. PMID: 19373240 Review. - Characterization of the 4C8 antigen involved in transendothelial migration of CD26(hi) T cells after tight adhesion to human umbilical vein endothelial cell monolayers.
Masuyama J, Yoshio T, Suzuki K, Kitagawa S, Iwamoto M, Kamimura T, Hirata D, Takeda A, Kano S, Minota S. Masuyama J, et al. J Exp Med. 1999 Mar 15;189(6):979-90. doi: 10.1084/jem.189.6.979. J Exp Med. 1999. PMID: 10075981 Free PMC article. - Conditional ablation of astroglial CCL2 suppresses CNS accumulation of M1 macrophages and preserves axons in mice with MOG peptide EAE.
Moreno M, Bannerman P, Ma J, Guo F, Miers L, Soulika AM, Pleasure D. Moreno M, et al. J Neurosci. 2014 Jun 11;34(24):8175-85. doi: 10.1523/JNEUROSCI.1137-14.2014. J Neurosci. 2014. PMID: 24920622 Free PMC article. - A survey of endogenous retrovirus (ERV) sequences in the vicinity of multiple sclerosis (MS)-associated single nucleotide polymorphisms (SNPs).
Brütting C, Emmer A, Kornhuber M, Staege MS. Brütting C, et al. Mol Biol Rep. 2016 Aug;43(8):827-36. doi: 10.1007/s11033-016-4004-0. Epub 2016 May 12. Mol Biol Rep. 2016. PMID: 27169423
References
- Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Investig Dermatol. 1992;99:683–690. - PubMed
- Ager A. Isolation and culture of high endothelial cells from rat lymph nodes. J Cell Sci. 1987;87:133–144. - PubMed
- Ager A, Mistry S. Interactions between lymphocytes and cultured high endothelial cells: an in vitro model of lymphocyte migration across high endothelial venule endothelium. Eur J Immunol. 1988;18:1265–1274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous