Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's disease brain - PubMed (original) (raw)
Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's disease brain
I Vincent et al. J Neurosci. 1997.
Abstract
We have shown previously that M-phase phospho-epitopes accumulate in neuronal tau proteins incorporated into the hallmark neurofibrillary tangles (NFT) of Alzheimer's disease (AD). In M phase, the epitopes are produced by cdc2/cyclin B1 kinase by a highly conserved mechanism believed to be quiescent in terminally differentiated neurons of adult brain. To determine whether an M-phase mechanism is possible in AD neurons, we first investigated the presence of cdc2 and cyclin B1 in AD. Both proteins were enriched in neurons with NFT and in neurons susceptible to NFT. An antibody specific for catalytically active cdc2 stained numerous NFT-containing neurons in AD but did not react with normal neurons. Double-labeling studies showed that active cdc2 and cyclin B1 coexist in AD neurons and co-localize with AD-specific mitotic phospho-epitopes. Mitotic kinase purified from AD and normal brain, using the yeast p13suc1 protein as affinity ligand, showed higher histone H1 phosphorylation activity in AD. Accordingly, the levels of cdc2 and cyclin B1 in p13suc1 fractions from AD were higher than normal. Consistent with a physiological relationship between NFT and mitotic kinase, NFT proteins co-purified with and became phosphorylated by the p13suc1-bound kinase in vitro. Furthermore, cdc2/cyclin B1 is the only one of several proline-directed kinases that created the TG/MC mitotic phospho-epitopes in recombinant tau in vitro. These findings suggest that aberrantly reexpressed cdc2/cyclin B1 in NFT-bearing neurons in AD brain contributes to the generation of M-phase phospho-epitopes in NFT.
Figures
Fig. 1.
Mitotic kinase activity is higher in AD than in normal brain. Normal and AD extracts were subjected to precipitation with p13suc1-agarose and the precipitates assayed for histone H-1 phosphorylation activity. Representative data from two sets of four normal and four AD cases are shown. Top panels show the histone H1 bands and, with the hippocampus, the position of the GST-p13suc1 fusion product as revealed by Coomassie blue staining. P13suc1-agarose and GST-p13suc1-agarose gave identical results and were used interchangeably. Bottom panels show the corresponding autoradiograms. Phosphorylation of H1 measured by Phosphoimager is higher in AD than in normal brain.
Fig. 2.
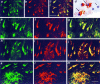
The cdc2 kinase is present in NFT-containing neurons in AD. Hippocampal tissue sections from AD (left panel), normal human (middle panel), and mouse brain (right panel) were stained with the indicated antibodies. Light micrographs of representative cases are shown. The TG-3 and MPM-2 rows demonstrate the specific occurrence of mitotic phospho-epitopes in AD neurons with NFT, with no similar staining in normal brain. NFT-bearing neurons are also stained with C-T cdc2 and PSTAIRE antibodies. In normal human and mouse brain, no neuronal staining is obvious with the cdc2 antibodies, but blood vessel endothelial cells are stained. Magnifications for the human and mouse illustrations are 150× and 75×, respectively.
Fig. 3.
Catalytically active cdc2 is present in NFT-containing neurons in AD. Hippocampal tissue sections from AD (left panel), normal human (middle panel), and mouse brain (right panel) were stained as indicated (active kinase,top and bottom rows; inactive kinase,middle row), and light micrographs of representative cases are shown. The active kinase antibody stains AD neurons with NFT and some blood vessels in normal brain. Neurites in neuritic plaques (NP, bottom row) are also positive with the active kinase antibody. Some AD cases (bottom row) showed weaker staining of glial cells with the active kinase antibody in addition to the marked reactivity with NFT. None of the NFT in these cases stained positive with the inactive kinase antibody (bottom row, inactive), although blood vessels and glia were stained. Magnifications for the top and_middle rows_ are 75×; for the NP, 400×; and for the remaining panels in the bottom row, 200×.
Fig. 4.
Cyclin B1 is present in neurons of AD brain. Hippocampal tissue sections from AD (top row), normal human (bottom row, left), and mouse brains (bottom row, right) were stained with human cyclin B1 monoclonal antibody. In some AD cases (AD1), staining of the neuronal cytoplasm and nucleus was observed, but in other cases, (AD2), primarily the neuronal nucleus (small arrows) and, occasionally, neurons (large arrow) were stained. Positive neuronal nuclei were also observed in some normal cases (shown), but not in mouse. Magnifications for the human and mouse illustrations are 150× and 75×, respectively.
Fig. 5.
Cdc2, cyclin B1, and TG-3/MPM-2 phospho-epitopes co-localize in AD neurons. Confocal micrographs illustrate AD hippocampus double-stained as follows: active kinase (a), cyclin B1 (b), and the merged image (c) showing co-localization of cyclin B1 with active kinase in neurons containing NFT; active kinase (e), C-T cdc2 (f), and the merged image (g) showing co-localization of C-T cdc2 with active kinase immunofluorescence; active kinase (h), MPM-2 (i), and the merged image (j) showing co-localization of active kinase and MPM-2 phospho-epitope; and active kinase (k), TG-3 (l), and the merged image (m) showing co-localization of active kinase and TG-3 phospho-epitope.d is a light micrograph showing double staining of AD hippocampus with primarily nuclear cyclin B1 staining (violet) and cytoplasmic active kinase (brown). Both proteins were found in the same neurons (large arrows), but some cyclin-positive neurons lacked active kinase immunoreactivity (small arrow). Scale bars: a–c, 12 μm; e–m, 17.6 μm. Magnification in d, 500×.
Fig. 6.
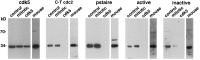
Cdc2 antibodies that stain AD neurons do not cross-react with cdk5. Immunoblot analysis was performed with the indicated antibodies and purified cdk5 (lane cdk5) or lysates from exponentially growing (control) and mitotic neuroblastoma cells and mouse lymphoma cells. Detection was by ECL with exposures of 20 sec to 3 min. The C-T cdc2, pstaire, active, and inactive kinase antibodies appropriately identified the 34 kDa cdc2 kinase in the human and mouse cell lysates. None of the cdc2 antibodies react with cdk5.
Fig. 7.
A, PHF–tau co-precipitates with Cdc2 and cyclin B1 in p13suc1 precipitates from brain. P13suc1 precipitates from four normal and four AD cases were analyzed by immunoblotting. The data are representative of 12 normal and 12 AD cases. Cdc2 was detected with CT-cdc2, PSTAIRE, and another cdc2-specific monoclonal antibody (shown). The amounts of cdc2 and cyclin B1 recovered in the p13suc1 precipitates from AD were higher than those from normal brain. Replicate blots were stained with the PHF-1 antibody to show the co-precipitation of PHF–tau in the AD p13suc1 precipitates. Similar staining of PHF–tau in the AD p13suc1 precipitates was seen with Alz-50, TG-3, and MC15 (data not shown). Only 1 of 12 normal cases showed PHF-1 immunoreactivity in the p13suc1 precipitate. This one case had hippocampal NFT when examined histopathologically. B, PHF–tau is phosphorylated by the active mitotic kinase complex in p13suc1 precipitates. P13suc1 precipitates from normal (Np) and AD (ADp) were analyzed by immunoblotting in relation to the starting extracts from which they were derived (Ns and_ADs_, respectively). Cdc2 and cyclin B1 are recovered in the p13suc1 precipitates in larger amounts in ADp relative to Np. The 62 kDa cyclin B1 is prominent in ADp but was evident in the other lanes as well, after longer ECL exposures. The 30 kDa cyclin B1-positive protein may be a degradation product of the 62 kDa cyclin. The nonmitotic cdk5 kinase is not recovered in the p13suc1 precipitates. A fraction of the total PHF-1-immunoreactive NFT protein in the AD extract (ADs) co-precipitates with the mitotic kinase complex (ADp). Incubation of the p13suc1 precipitates with γ-32P-labeled ATP resulted in marked incorporation of labeled phosphate into NFT protein in the ADp.
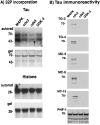
Fig. 8.
Preferential creation of the TG/MC phospho-epitopes in recombinant tau by cdc2/cyclin B. A,32P incorporation. Histone H1 and tau were incubated with the indicated proline-directed kinases. Bottom panels(i.e., gel) for each substrate show Coomassie blue staining of the protein in the gel, and the top panels (i.e., autorad) show the corresponding autoradiograms. Equivalent amounts of32P were incorporated into H1 after incubation with the indicated kinases. This was also the case with tau as substrate, except that higher amounts of 32P incorporation were observed with MAPK. The positions of molecular weight (MW) markers in kilodaltons are shown on the left of each panel. B, Tau immunoreactivity. Replicate panels of tau protein phosphorylated with MAPK, cdc2/cyclin B, cdk5, or GSK-3, respectively, were stained with the TG/MC and PHF-1 antibodies as indicated on the left_of each panel. Whereas all the kinases produced the PHF-1 phospho-epitope in tau, only cdc2/cyclin B produced the mitotic TG/MC epitopes. The position of the 70 kDa MW marker is shown on the_left of each panel.
Similar articles
- Constitutive Cdc25B tyrosine phosphatase activity in adult brain neurons with M phase-type alterations in Alzheimer's disease.
Vincent I, Bu B, Hudson K, Husseman J, Nochlin D, Jin L. Vincent I, et al. Neuroscience. 2001;105(3):639-50. doi: 10.1016/s0306-4522(01)00219-6. Neuroscience. 2001. PMID: 11516829 - Mitotic epitopes are incorporated into age-dependent neurofibrillary tangles in Niemann-Pick disease type C.
Zhang M, Wang X, Jiang F, Wang W, Vincent I, Bu B. Zhang M, et al. Brain Pathol. 2010 Mar;20(2):367-77. doi: 10.1111/j.1750-3639.2009.00286.x. Epub 2009 May 20. Brain Pathol. 2010. PMID: 19476463 Free PMC article. - Cdc2 phosphorylation of nucleolin demarcates mitotic stages and Alzheimer's disease pathology.
Dranovsky A, Vincent I, Gregori L, Schwarzman A, Colflesh D, Enghild J, Strittmatter W, Davies P, Goldgaber D. Dranovsky A, et al. Neurobiol Aging. 2001 Jul-Aug;22(4):517-28. doi: 10.1016/s0197-4580(00)00248-7. Neurobiol Aging. 2001. PMID: 11445251 - Physiology and pathology of tau protein kinases in relation to Alzheimer's disease.
Imahori K, Uchida T. Imahori K, et al. J Biochem. 1997 Feb;121(2):179-88. J Biochem. 1997. PMID: 9089387 Review. - Aberrant protein phosphorylation and cytoarchitecture in Alzheimer's disease.
Saitoh T, Iimoto D. Saitoh T, et al. Prog Clin Biol Res. 1989;317:769-80. Prog Clin Biol Res. 1989. PMID: 2690121 Review.
Cited by
- The genomically mosaic brain: aneuploidy and more in neural diversity and disease.
Bushman DM, Chun J. Bushman DM, et al. Semin Cell Dev Biol. 2013 Apr;24(4):357-69. doi: 10.1016/j.semcdb.2013.02.003. Epub 2013 Mar 4. Semin Cell Dev Biol. 2013. PMID: 23466288 Free PMC article. Review. - Deregulation of cdk5, hyperphosphorylation, and cytoskeletal pathology in the Niemann-Pick type C murine model.
Bu B, Li J, Davies P, Vincent I. Bu B, et al. J Neurosci. 2002 Aug 1;22(15):6515-25. doi: 10.1523/JNEUROSCI.22-15-06515.2002. J Neurosci. 2002. PMID: 12151531 Free PMC article. - Oxidative imbalance in Alzheimer's disease.
Zhu X, Lee HG, Casadesus G, Avila J, Drew K, Perry G, Smith MA. Zhu X, et al. Mol Neurobiol. 2005;31(1-3):205-17. doi: 10.1385/MN:31:1-3:205. Mol Neurobiol. 2005. PMID: 15953822 Review. - Cell cycle activation and spinal cord injury.
Wu J, Stoica BA, Faden AI. Wu J, et al. Neurotherapeutics. 2011 Apr;8(2):221-8. doi: 10.1007/s13311-011-0028-2. Neurotherapeutics. 2011. PMID: 21373950 Free PMC article. Review. - Core cell cycle machinery is crucially involved in both life and death of post-mitotic neurons.
Marlier Q, D'aes T, Verteneuil S, Vandenbosch R, Malgrange B. Marlier Q, et al. Cell Mol Life Sci. 2020 Nov;77(22):4553-4571. doi: 10.1007/s00018-020-03548-1. Epub 2020 May 31. Cell Mol Life Sci. 2020. PMID: 32476056 Free PMC article. Review.
References
- Azzi L, Meijer L, Ostvold A-C, Lew J, Wang JH. Purification of a 15 kDa cdk4- and cdk5- binding protein. J Biol Chem. 1994;269:13279–13288. - PubMed
- Braak H, Braak E. Neuropathologic staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
- Clarke AR, Maandag ER, van Roon M, van der Lugt NM, van der Valk M, Hooper ML, Berns A, te Riele H. Requirement for a functional Rb-1 gene in murine development. Nature. 1992;359:270–271. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous