TrkB signaling is required for postnatal survival of CNS neurons and protects hippocampal and motor neurons from axotomy-induced cell death - PubMed (original) (raw)
TrkB signaling is required for postnatal survival of CNS neurons and protects hippocampal and motor neurons from axotomy-induced cell death
S Alcántara et al. J Neurosci. 1997.
Abstract
Newborn mice carrying targeted mutations in genes encoding neurotrophins or their signaling Trk receptors display severe neuronal deficits in the peripheral nervous system but not in the CNS. In this study, we show that trkB (-/-) mice have a significant increase in apoptotic cell death in different regions of the brain during early postnatal life. The most affected region in the brain is the dentate gyrus of the hippocampus, although elevated levels of pyknotic nuclei were also detected in cortical layers II and III and V and VI, the striatum, and the thalamus. Furthermore, axotomized hippocampal and motor neurons of trkB (-/-) mice have significantly lower survival rates than those of wild-type littermates. These results suggest that neurotrophin signaling through TrkB receptors plays a role in the survival of CNS neurons during postnatal development. Moreover, they indicate that TrkB receptor signaling protects subpopulations of CNS neurons from injury- and axotomy-induced cell death.
Figures
Fig. 1.
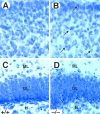
Cytoarchitecture of brain structures of postnatal_trk_B (−/−) mutant mice. A–D, Low-power micrographs of the brain of wild-type (A, C) and_trk_B (−/−) (B, D) mice. Notice the normal appearance of Nissl-stained brain sections from P13 (B) and P16 (D) _trk_B (−/−) mice compared with their age-mated control littermates (A, C, respectively). E, F, High-power magnification of the neocortex of P16 wild-type (E) and_trk_B (−/−) (F) mice. Notice the presence of empty areas devoid of neurons in _trk_B (−/−) mice (F) (from D, inset).G, H, Calretinin immunoreactivity in the neocortex of P10 wild-type (G) and trk_B (−/−) (H) mice. Cortical layers are denoted by_roman numerals. NC, Neocortex;H, hippocampus; T, thalamus. Magnification: A–D, 2.5×; E, F, 40×;G, H, 10×.
Fig. 2.
Increased cell death in _trk_B (−/−) mice. A–D, Cresyl violet-stained sections of the neocortex (A, B) and dentate gyrus (C, D) of P16 control (A, C) and_trk_B (−/−) (B, D) mice. Notice the presence of pyknotic nuclei in layers II and III of the neocortex (B, arrows) and the granular layer of the dentate gyrus (D) in the _trk_B (−/−) mutant mice.GL, Granular layer; H, hilus;ML, molecular layer. Magnification, 40×.
Fig. 3.
Neurons in _trk_B (−/−) mice die by apoptosis. A, B, Immunolabeling with c-Jun antibody reveals the presence of pyknotic cells in layers II and III (A) and degenerating neurons in the pyriform cortex (B) in P10 _trk_B (−/−) mice.C, Electron micrograph showing the characteristic morphology of an apoptotic cell in the dentate gyrus that is being engulfed by a neighboring cell in a _trk_B (−/−) mouse.D–G, TUNEL staining of neocortex (layers II and III) (D, E) and hippocampus (F, G); sections from P13 control (D, F) and _trk_B (−/−) (E, G) mice. Notice the increase in TUNEL-positive nuclei in the trk_B (−/−) mice.Arrows in A denote pyknotic nuclei. The_arrowhead denotes a pyknotic nucleus negative for c-Jun staining. Sections in A and B are counterstained with cresyl violet. GL, Granular layer;H, hilus; ML, molecular layer. Magnification: A, B, 30×; C, 15,000×;D–G, 40×.
Fig. 4.
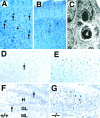
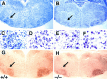
Expression of calcium-binding proteins in degenerating neurons in _trk_B (−/−) mutant mice.A–D, Calretinin immunoreactivity in layers II and III of the neocortex (A, B, D) and the stratum oriens of the hippocampus (C) of P10 _trk_B (−/−) mutant mice. Presumably, degenerating neurons with atrophic somata and beaded dendrites are immunoreactive for calretinin staining (A, B, arrows). E, Calbindin expression in layers II and III of the neocortex of a P13 _trk_B (−/−) mice. Arrows in C–E denote pyknotic cells immunoreactive for different calcium-binding proteins.Arrowheads denote unlabeled pyknotic cells. Sections are counterstained with cresyl violet. Magnification: _A, B,_35×; C, D, 40×; E, 45×.
Fig. 5.
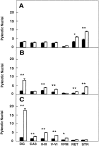
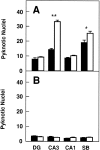
Number of pyknotic nuclei in different regions of the brain at several developmental stages (expressed as number per 100,000 μm2). A, P5–P8 mice (control mice, n = 4; _trk_B (−/−) mice,n = 4). B, P10–P12 mice (control mice, n = 6; _trk_B (−/−) mice,n = 7). C, P13–P18 mice (control mice, n = 10; _trk_B (−/−) mice,n = 11). Black bars, Control mice;_white bars, trk_B (−/−) mutant mice. _DG,_Dentate gyrus; CA3, CA3 hippocampal field;II–III and V–VI, cortical layers II and III and V and VI, respectively; VPM, ventroposterior medialis thalamic nucleus; RET, reticular thalamic nucleus; STR, striatum. Error bars indicate SEM;asterisks indicate that there are significant differences between control and _trk_B (−/−) mutant mice (*p < 0.01; **p < 0.001).
Fig. 6.
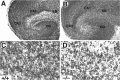
Increased cell death in hippocampal slice cultures from _trk_B (−/−) mice. A, B, Cresyl violet-stained sections show the normal structure of hippocampal slices in both control (A, C) and _trk_B (−/−) mice (B, D) after 3 d in culture. C, D, Higher-magnification micrographs show an increase in pyknotic nuclei in the CA3 region of _trk_B (−/−) mutant mice (D) when compared with control mice (C). CA1, Pyramidal layer CA1;CA3, pyramidal layer CA3; DG, dentate gyrus; SB, subiculum. Magnification: A, B, 10×; C, D, 40×.
Fig. 7.
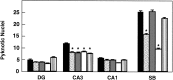
Number of pyknotic nuclei in different regions of the hippocampus after 3 (A) and 5 (B) d in culture (expressed as number per 25,000 μm2).Black bars, Control mice (n = 8 in each group of cultures); white bars, trk_B (−/−) mutant mice (n = 8 in each group of cultures).DG, Dentate gyrus; CA1 and_CA3, CA1 and CA3 hippocampal fields, respectively;SB, subiculum. Error bars indicate SEM;asterisks indicate that there are significant differences between control and _trk_B (−/−) mutant mice (*p < 0.001; **p < 0.0001).
Fig. 8.
Number of pyknotic nuclei in different regions of the hippocampus after 3 d of treatment with neurotrophins in culture (expressed as number per 25,000 μm2).Black bars, Vehicle; dotted bars, BDNF;gray bars, NT-3; white bars, NT-4;striped bars, NGF (n = 8 or 9 cultures per group). DG, Dentate gyrus;CA1 and CA3, CA1 and CA3 hippocampal fields, respectively; SB, subiculum. Error bars indicate SEM; asterisks indicate that there are significant differences between the different treatments (*p < 0.01, ANOVA with Fisher’s protected least significant difference for_post hoc_ comparisons).
Fig. 9.
Decrease in the number of facial motor neurons after axotomy in _trk_B (−/−) mice. _A–F,_Cresyl violet-stained sections show the reduction in the size of the axotomized facial motor nucleus (B) but the normal appearance of the surviving motor neurons (E) in P10_trk_B (−/−) mice. A, C, D, Wild-type mice. _B, E, F, trk_B (−/−) mice. _G, H,_Acetylcholinesterase activity in P7 wild-type (G) and_trk_B (−/−) (H) mice. Similar activity was observed in the _trk_B (−/−) mutant mice when compared with wild-type mice. Magnification: A, B, G, H, 2.5×; C–F, 100×.
Similar articles
- TrkB and TrkC neurotrophin receptors cooperate in promoting survival of hippocampal and cerebellar granule neurons.
Minichiello L, Klein R. Minichiello L, et al. Genes Dev. 1996 Nov 15;10(22):2849-58. doi: 10.1101/gad.10.22.2849. Genes Dev. 1996. PMID: 8918886 - TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections.
Martínez A, Alcántara S, Borrell V, Del Río JA, Blasi J, Otal R, Campos N, Boronat A, Barbacid M, Silos-Santiago I, Soriano E. Martínez A, et al. J Neurosci. 1998 Sep 15;18(18):7336-50. doi: 10.1523/JNEUROSCI.18-18-07336.1998. J Neurosci. 1998. PMID: 9736654 Free PMC article. - Severe sensory deficits but normal CNS development in newborn mice lacking TrkB and TrkC tyrosine protein kinase receptors.
Silos-Santiago I, Fagan AM, Garber M, Fritzsch B, Barbacid M. Silos-Santiago I, et al. Eur J Neurosci. 1997 Oct;9(10):2045-56. doi: 10.1111/j.1460-9568.1997.tb01372.x. Eur J Neurosci. 1997. PMID: 9421165 - Role of neurotrophins in mouse neuronal development.
Klein R. Klein R. FASEB J. 1994 Jul;8(10):738-44. doi: 10.1096/fasebj.8.10.8050673. FASEB J. 1994. PMID: 8050673 Review. - The Trk family of neurotrophin receptors.
Barbacid M. Barbacid M. J Neurobiol. 1994 Nov;25(11):1386-403. doi: 10.1002/neu.480251107. J Neurobiol. 1994. PMID: 7852993 Review.
Cited by
- Bispecific antibody shuttles targeting CD98hc mediate efficient and long-lived brain delivery of IgGs.
Pornnoppadol G, Bond LG, Lucas MJ, Zupancic JM, Kuo YH, Zhang B, Greineder CF, Tessier PM. Pornnoppadol G, et al. Cell Chem Biol. 2024 Feb 15;31(2):361-372.e8. doi: 10.1016/j.chembiol.2023.09.008. Epub 2023 Oct 26. Cell Chem Biol. 2024. PMID: 37890480 - Involvement of brain-derived neurotrophic factor signaling in the pathogenesis of stress-related brain diseases.
Numakawa T, Kajihara R. Numakawa T, et al. Front Mol Neurosci. 2023 Sep 14;16:1247422. doi: 10.3389/fnmol.2023.1247422. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37781095 Free PMC article. Review. - Central Facial Nervous System Biomolecules Involved in Peripheral Facial Nerve Injury Responses and Potential Therapeutic Strategies.
Lee JM, Choi YJ, Yoo MC, Yeo SG. Lee JM, et al. Antioxidants (Basel). 2023 May 1;12(5):1036. doi: 10.3390/antiox12051036. Antioxidants (Basel). 2023. PMID: 37237902 Free PMC article. Review. - Muscular Swedish mutant APP-to-Brain axis in the development of Alzheimer's disease.
Pan JX, Lee D, Sun D, Zhao K, Xiong L, Guo HH, Ren X, Chen P, Lopez de Boer R, Lu Y, Lin H, Mei L, Xiong WC. Pan JX, et al. Cell Death Dis. 2022 Nov 10;13(11):952. doi: 10.1038/s41419-022-05378-4. Cell Death Dis. 2022. PMID: 36357367 Free PMC article. - A Brief Overview on BDNF-Trk Pathway in the Nervous System: A Potential Biomarker or Possible Target in Treatment of Multiple Sclerosis?
Schirò G, Iacono S, Ragonese P, Aridon P, Salemi G, Balistreri CR. Schirò G, et al. Front Neurol. 2022 Jul 12;13:917527. doi: 10.3389/fneur.2022.917527. eCollection 2022. Front Neurol. 2022. PMID: 35911894 Free PMC article. Review.
References
- Altman J, Bayer SA. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol. 1990a;301:325–342. - PubMed
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990b;301:365–381. - PubMed
- Arenas E, Persson H. Neurotrophin-3 prevents the death of adult central noradrenergic neurons in vivo. Nature. 1994;367:368–371. - PubMed
- Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. - PubMed
- Bayer SA. Development of the hippocampal region of the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol. 1980;190:87–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases