Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulates interleukin 2 production - PubMed (original) (raw)
Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulates interleukin 2 production
A J da Silva et al. Proc Natl Acad Sci U S A. 1997.
Abstract
T cell receptor zeta (TcRzeta)/CD3 ligation initiates a signaling cascade that involves src kinases p56(lck) and zeta-associated protein 70, leading to the phosphorylation of substrates such as TcRzeta, Vav, SH2-domain-containing leukocyte protein 76 (SLP-76), cbl, and p120/130. FYN binding protein (FYB or p120/130) associates with p59(fyn), the TcRzeta/CD3 complex, and becomes tyrosine-phosphorylated in response to receptor ligation. In this study, we report the cDNA cloning of human and murine FYB and show that it is restricted in expression to T cells and myeloid cells and possesses an overall unique hydrophilic sequence with several tyrosine-based motifs, proline-based type I and type II SH3 domain binding motifs, several putative lysine/glutamic acid-rich nuclear localization motifs, and a SH3-like domain. In addition to binding the src kinase p59(fyn), FYB binds specifically to the hematopoietic signaling protein SLP-76, an interaction mediated by the SLP-76 SH2 domain. In keeping with this, expression of FYB augmented interleukin 2 secretion from a T cell hybridoma, DC27.10, in response to TcRzeta/CD3 ligation. FYB is therefore a novel hematopoietic protein that acts as a component of the FYN and SLP-76 signaling cascades in T cells.
Figures
Figure 1
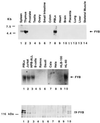
Sequence and structural motifs of FYB. (A) Overall amino acid sequence of human (upper sequence) and murine (lower sequence) FYB cDNAs. Representation depicts overall structure and the location of potential motifs that include a proline-rich region followed by a YEDI motif, two putative lysine/glutamic acid/arginine-rich nuclear localization motifs, and a SH3-like domain. Amino acid sequences of human and murine FYB (783 and 774 residues, respectively) show the position of the type I and type II proline-rich SH3 domain recognition motifs (underlined with bold line), the YEDI motif (indicated within shaded box), putative nuclear localization motifs (underlined with double line), and the C-terminal SH3-like domain (indicated within open box). (B) Schematic comparison of the FYB SH3-like domain with other SH3 domains. SH3-like domain shows particular homology to the SH3 domains of the protein-tyrosine kinases such as B lymphocyte kinase, p60yes, Bruton’s/agammaglobulinemia tyrosine kinase, and CRK-like protein (CRKL). Similarity is also seen with SH3 domain of a C. elegans SH3-carrying-protein and a Drosophila SH2/SH3 adaptor protein. Conserved SH3 domain residues include the βa region valine (residue 707), a RT loop tyrosine (residue 709), aspartic acid (residue 726), and a glycine (residue 732), a βd region glycine-tyrosine (residues 756–757), and a tyrosine (residue 762) proximal to βe region. (C) FYB cDNA clone generates polypeptide recognized by anti-FYB antiserum. HA-tagged FYB cDNA was expressed in COS (lane 2) and DC27.10 (lane 6) cells by using electroporation. Cell lysates were subjected to immunoprecipitation using anti-HA (lanes 1, 2, 5, and 6) or anti-FYB (lane 4) and precipitates were immunoblotted with anti-FYB serum.
Figure 2
Expression of FYB is restricted to T and myeloid cells. (Top) Northern blot hybridization with the human FYB cDNA clone against CLONTECH poly(A)+ RNA purified from spleen (lane 1), thymus (lane 2), prostate (lane 3), testis (lane 4), ovary (lane 5), small intestine (lane 6), colon (lane 7), peripheral blood lymphocytes (lane 8), heart (lane 9), brain (lane 10), placenta (lane 11), lung (lane 12), liver (lane 13), and skeletal muscle (lane 14). (Middle) Hybridization of FYB cDNA against total RNA purified from human peripheral blood lymphocyte-purified T cells (lane 1), Jurkat cells (lane 2), HPB-ALL cells (lane 3), tonsil-purified B-cells (lane 4), Ramos cells (lane 5), Daudi cells (lane 6), lung cell line Cala (lane 7), uterine cell line Hela (lane 8), breast cell line HLB-100 (lane 9), and myeloid cell line HL-60 (lane 10). (Lower) Parallel immunoblot analysis of cells from Middle with anti-FYB antiserum.
Figure 3
FYB and p59fyn(T) associate in COS cells. COS cells were transfected with pSRα vector control (lanes 1, 5, 9, and 13), pSRα-FYB(Ha) (lanes 2, 6, 10, and 14), pSRαFyn (lanes 3, 7, 11, and 15), or pSRα-FYB(Ha) and pSRαFyn (lanes 4, 8, 12, and 16). Cell lysates were incubated with anti-Ha mAb to precipitate FYB or with anti-fyn as indicated, precipitates were separated by SDS/PAGE, and samples were analyzed with anti-phosphotyrosine (lanes 1–8), anti-FYB (lanes 9–12), or anti-Fyn (lanes 13–16).
Figure 4
FYB associates with SLP-76 SH2 in T cells. (A) FYB associates with SLP-76. Lysates from DC27.10 cells were subjected to precipitation with preimmune (lanes 1 and 3), anti-SLP-76 (lanes 2 and 4), anti-FYB (lane 5), or GST (lanes 6 and 8) and GST–FYB (lanes 7 and 9) fusion proteins. Precipitates were then analyzed by immunoblotting with anti-phosphotyrosine mAb 4G10 (lanes 1, 2, 6, and 7), anti-FYB (lanes 3–5), or anti-SLP-76 (lanes 8 and 9). (B) SLP-76 SH2 domain specifically recognizes FYB from T cells. DC27.10-derived lysates were incubated with GST fusion proteins as indicated: GST alone (lane 1), GRB-2 (lane 2), SH2-p85 (lane 3), SH2-HCP (lane 4), SH2-SYP (lane 5), SH2-SLP-76 (lane 6), and SH2-FYN (lane 7). Precipitates were separated by SDS/PAGE and analyzed by immunoblotting with anti-FYB. (C) SLP-76 SH2 domain preferentially binds to FYB from T cells. Cell lysates from DC27.10 cells (lanes 1–4) and Jurkat (lanes 5–8) were incubated with GST (lanes 1, 3, 5, and 7) or GST–SH2-SLP-76 fusion protein (lanes 2, 4, 6, and 8) and subjected to immunoblotting analysis with anti-phosphotyrosine (lanes 1, 2, 5, and 6) or anti-FYB (lanes 3, 4, 7, and 8) serum.
Figure 5
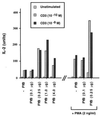
FYB up-regulates TcRζ/CD3-induced IL-2 production. FYB transfected DC27.10 cells showed a 3- to 5-fold augmentation of IL-2 production in response to anti-CD3 alone or anti-CD3 plus phorbol ester. FYB cDNA (at 0.1, 0.25, 1.0, or 4.0 μg per 106 cells) or mock-transfected DC27.10 cells (32) were incubated overnight with medium alone, anti-CD3, or anti-CD3 mAb plus phorbol 12-myristate 13-acetate, as indicated. Cell culture supernatants were assayed for IL-2 by a bioassay using CTLL-2 cells.
Similar articles
- FYB (FYN binding protein) serves as a binding partner for lymphoid protein and FYN kinase substrate SKAP55 and a SKAP55-related protein in T cells.
Liu J, Kang H, Raab M, da Silva AJ, Kraeft SK, Rudd CE. Liu J, et al. Proc Natl Acad Sci U S A. 1998 Jul 21;95(15):8779-84. doi: 10.1073/pnas.95.15.8779. Proc Natl Acad Sci U S A. 1998. PMID: 9671755 Free PMC article. - Novel isoform of lymphoid adaptor FYN-T-binding protein (FYB-130) interacts with SLP-76 and up-regulates interleukin 2 production.
Veale M, Raab M, Li Z, da Silva AJ, Kraeft SK, Weremowicz S, Morton CC, Rudd CE. Veale M, et al. J Biol Chem. 1999 Oct 1;274(40):28427-35. doi: 10.1074/jbc.274.40.28427. J Biol Chem. 1999. PMID: 10497204 - Molecular analysis of the fyn-complex: cloning of SKAP55 and SLAP-130, two novel adaptor proteins which associate with fyn and may participate in the regulation of T cell receptor-mediated signaling.
Schraven B, Marie-Cardine A, Koretzky G. Schraven B, et al. Immunol Lett. 1997 Jun 1;57(1-3):165-9. doi: 10.1016/s0165-2478(97)00053-9. Immunol Lett. 1997. PMID: 9232446 Review. No abstract available. - The FRK/RAK-SHB signaling cascade: a versatile signal-transduction pathway that regulates cell survival, differentiation and proliferation.
Annerén C, Lindholm CK, Kriz V, Welsh M. Annerén C, et al. Curr Mol Med. 2003 Jun;3(4):313-24. doi: 10.2174/1566524033479744. Curr Mol Med. 2003. PMID: 12776987 Review.
Cited by
- Structural requirements of SLP-76 in signaling via the high-affinity immunoglobulin E receptor (Fc epsilon RI) in mast cells.
Kettner A, Pivniouk V, Kumar L, Falet H, Lee JS, Mulligan R, Geha RS. Kettner A, et al. Mol Cell Biol. 2003 Apr;23(7):2395-406. doi: 10.1128/MCB.23.7.2395-2406.2003. Mol Cell Biol. 2003. PMID: 12640123 Free PMC article. - Synergistic regulation of immunoreceptor signaling by SLP-76-related adaptor Clnk and serine/threonine protein kinase HPK-1.
Yu J, Riou C, Davidson D, Minhas R, Robson JD, Julius M, Arnold R, Kiefer F, Veillette A. Yu J, et al. Mol Cell Biol. 2001 Sep;21(18):6102-12. doi: 10.1128/MCB.21.18.6102-6112.2001. Mol Cell Biol. 2001. PMID: 11509653 Free PMC article. - Regulation of hematopoietic cell development and activation by adapter proteins.
Koretzky GA, Abtahian F, Derimanov GS, Dmowski SA, Guerriero A, Jordan MS, Maltzman JS, Olenchock BA, Singer AL, Wu JN, Zhong XP. Koretzky GA, et al. Immunol Res. 2003;27(2-3):357-66. doi: 10.1385/IR:27:2-3:357. Immunol Res. 2003. PMID: 12857981 Review. - The ADAP/SKAP55 signaling module regulates T-cell receptor-mediated integrin activation through plasma membrane targeting of Rap1.
Kliche S, Breitling D, Togni M, Pusch R, Heuer K, Wang X, Freund C, Kasirer-Friede A, Menasche G, Koretzky GA, Schraven B. Kliche S, et al. Mol Cell Biol. 2006 Oct;26(19):7130-44. doi: 10.1128/MCB.00331-06. Mol Cell Biol. 2006. PMID: 16980616 Free PMC article. - SLP-65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation.
Wienands J, Schweikert J, Wollscheid B, Jumaa H, Nielsen PJ, Reth M. Wienands J, et al. J Exp Med. 1998 Aug 17;188(4):791-5. doi: 10.1084/jem.188.4.791. J Exp Med. 1998. PMID: 9705962 Free PMC article.
References
- Rudd C E, Janssen O, Cai Y C, da Silva A J, Raab M, Prasad K V. Immunol Today. 1994;15:225–234. - PubMed
- Mustelin T. Immunity. 1994;1:351–356. - PubMed
- Wange R L, Samelson L E. Immunity. 1996;5:197–205. - PubMed
- Chan A C, Iwashima M, Turck C W, Weiss A. Cell. 1992;71:649–662. - PubMed
- Iwashima M, Irving B A, van Oers N S C, Chan A C, Weiss A. Science. 1994;263:1136–1139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous