Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice - PubMed (original) (raw)
Comparative Study
Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice
D J Liebl et al. J Neurosci. 1997.
Abstract
Gene-targeting experiments of Trk receptors and neurotrophins has confirmed the expectation that embryonic sensory and sympathetic neurons require neurotrophin function for survival. They have further revealed correlation between a specific neurotrophin requirement and eventual sensory modality. We have analyzed embryonic and neonatal mice with mutations in the BDNF, neurotrophin 3 (NT-3), and TrkC genes. Our data confirm an unexpectedly high proportion of sensory neuron losses in NT-3 (>70%), BDNF (>20%), and TrkC (>30%) mutants, which encompass populations thought to be NGF-dependent. Direct comparison of TrkC and NT-3 mutants indicates that only a subset of the NT-3-dependent neurons also requires TrkC. The observed losses in our TrkC mutant, which is null for all proteins encoded by the gene, are more severe than those previously reported for the kinase-negative TrkC mutation, implicating additional and important functions for the truncated receptors. Our data further indicate that mature NGF-requiring neurons undergo precocious and transitory requirements for NT-3 and/or BDNF. We suggest that neurotrophins may function in creating early heterogeneity that would enable ganglia to compensate for diverse modality requirements before the period of naturally occurring death.
Figures
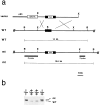
Fig. 1.
Generation of BDNF mutant mice. a, Schematic showing the replacement vector and strategy used to inactivate the BDNF gene. The filled bar indicates the BDNF coding region. Restriction enzyme sites are as indicated:B, _Bgl_II; E,_Eco_Rl; S, _Sma_l;X, _Xba_l. b, Southern blot analysis of tail DNA from a litter obtained intercrossing two BDNF +/− mice. _Bgl_II restriction enzyme digestion and the 5′ external probe indicated in a were used to detect rearrangement in the mouse BDNF locus. The 14 kb wild-type (WT) and 15.6 kb rearranged (mt) DNA bands are indicated.
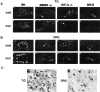
Fig. 2.
TrkB (A) and TrkC (B) expression in trigeminal gangila (TG) and dorsal root ganglia (DRG) of wild-type and BDNF and/or NT-3 mutant neonates. q and_r_ show representative examples of positive and negative Trk-expressing trigeminal and DRG neurons, respectively. In a–p arrows indicate small groups of TrkB- or TrkC-expressing neurons still present in mutant mice. In q and r black arrowheads indicate “positive” Trk-expressing neurons; white arrowheads indicate negative Trk-expressing neurons.
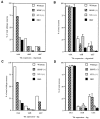
Fig. 3.
Quantitation of TrkA-, B-, and C-expressing neurons in the TG (A, B) and DRG (C, D) of neonatal mice. A, C, Percentage of neurons expressing Trk receptor mRNA compared with the total number of neurons present in wild-type ganglia. B,D, Relative percentage of neurons expressing Trk mRNA as a function of the total number of neurons in their respective ganglia.Open bar, Wild type; hatched bar, BDNF−/−; dotted bar, NT-3−/−; solid bar, BDNF/NT-3 DKO. Data and statistical analysis are from Table 1.
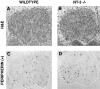
Fig. 4.
Analysis of peripherin-positive L4 DRG neurons of wild-type (A, C) and NT-3 mutant (B, D) E13–E13.25 embryos. Hematoxylin and eosin (H&E)-stained sections (A, B) were used for quantification of total cell numbers, whereas antibodies to peripherin (B, D) identified neurons. The ganglia are outlined by a dotted line.
Fig. 5.
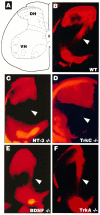
Ia afferents are absent in TrkC and NT-3 mutants but not in BDNF or TrkA mutants. Hemisection view of DiI (see Materials and Methods) retrogradely labeled sensory afferents and motor neurons in the P0.5 spinal cord. A, Schematic diagram of the spinal cord indicating the dorsal horn (DH) and associated afferent laminae. The ventral horn (VH) indicates where motoneuron nuclei are retrogradely labeled. B–F, Samples of cords from mutant pups as indicated. The arrowheads point to the location of Ia afferents.
Fig. 6.
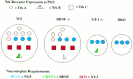
Schematic diagram of dorsal root ganglion neuronal subpopulations present in newborn animals as a consequence of neurotrophin knock-outs. In wild-type ganglia (WT), the red stippled circles are presumed to be TrkA- and NGF-dependent neurons based on other studies (Crowley et al., 1994; Smeyne et al., 1994) and as undergoing transitory NT-3 requirements based on the present study and others (Tessarollo et al., 1994; Airaksinen et al., 1996; Fariñas et al., 1996; White et al., 1996). Shape indicates receptor type (circle, TrkA; triangle, TrkB;square, TrkC). Color indicates neurotrophin requirements (blue, NGF;green, BDNF; red, NT-3), and the relative numbers indicate approximate percentages.
Similar articles
- Characterization of neurotrophin and Trk receptor functions in developing sensory ganglia: direct NT-3 activation of TrkB neurons in vivo.
Fariñas I, Wilkinson GA, Backus C, Reichardt LF, Patapoutian A. Fariñas I, et al. Neuron. 1998 Aug;21(2):325-34. doi: 10.1016/s0896-6273(00)80542-5. Neuron. 1998. PMID: 9728914 Free PMC article. - Differential dependency of cutaneous mechanoreceptors on neurotrophins, trk receptors, and P75 LNGFR.
Fundin BT, Silos-Santiago I, Ernfors P, Fagan AM, Aldskogius H, DeChiara TM, Phillips HS, Barbacid M, Yancopoulos GD, Rice FL. Fundin BT, et al. Dev Biol. 1997 Oct 1;190(1):94-116. doi: 10.1006/dbio.1997.8658. Dev Biol. 1997. PMID: 9331334 - Developmental changes in NT3 signalling via TrkA and TrkB in embryonic neurons.
Davies AM, Minichiello L, Klein R. Davies AM, et al. EMBO J. 1995 Sep 15;14(18):4482-9. doi: 10.1002/j.1460-2075.1995.tb00127.x. EMBO J. 1995. PMID: 7556091 Free PMC article. - Role of neurotrophins and trk receptors in the development and maintenance of sensory neurons: an overview.
Lindsay RM. Lindsay RM. Philos Trans R Soc Lond B Biol Sci. 1996 Mar 29;351(1338):365-73. doi: 10.1098/rstb.1996.0030. Philos Trans R Soc Lond B Biol Sci. 1996. PMID: 8730773 Review. - Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance.
Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Fritzsch B, et al. Prog Brain Res. 2004;146:265-78. doi: 10.1016/S0079-6123(03)46017-2. Prog Brain Res. 2004. PMID: 14699969 Review.
Cited by
- Transsynaptic Tracing from Taste Receptor Cells Reveals Local Taste Receptor Gene Expression in Gustatory Ganglia and Brain.
Voigt A, Bojahr J, Narukawa M, Hübner S, Boehm U, Meyerhof W. Voigt A, et al. J Neurosci. 2015 Jul 1;35(26):9717-29. doi: 10.1523/JNEUROSCI.0381-15.2015. J Neurosci. 2015. PMID: 26134654 Free PMC article. - Stem cell therapy for the inner ear: recent advances and future directions.
Okano T, Kelley MW. Okano T, et al. Trends Amplif. 2012 Mar;16(1):4-18. doi: 10.1177/1084713812440336. Epub 2012 Apr 17. Trends Amplif. 2012. PMID: 22514095 Free PMC article. Review. - Effects of brain-derived neurotrophic factor on dopaminergic function and motor behavior during aging.
Boger HA, Mannangatti P, Samuvel DJ, Saylor AJ, Bender TS, McGinty JF, Fortress AM, Zaman V, Huang P, Middaugh LD, Randall PK, Jayanthi LD, Rohrer B, Helke KL, Granholm AC, Ramamoorthy S. Boger HA, et al. Genes Brain Behav. 2011 Mar;10(2):186-98. doi: 10.1111/j.1601-183X.2010.00654.x. Epub 2010 Oct 19. Genes Brain Behav. 2011. PMID: 20860702 Free PMC article. - Atrial natriuretic peptide type C induces a cell-cycle switch from proliferation to differentiation in brain-derived neurotrophic factor- or nerve growth factor-primed olfactory receptor neurons.
Simpson PJ, Miller I, Moon C, Hanlon AL, Liebl DJ, Ronnett GV. Simpson PJ, et al. J Neurosci. 2002 Jul 1;22(13):5536-51. doi: 10.1523/JNEUROSCI.22-13-05536.2002. J Neurosci. 2002. PMID: 12097505 Free PMC article. - An intrastriatal brain-derived neurotrophic factor infusion restores striatal gene expression in Bdnf heterozygous mice.
Saylor AJ, McGinty JF. Saylor AJ, et al. Brain Struct Funct. 2010 Aug;215(2):97-104. doi: 10.1007/s00429-010-0282-9. Epub 2010 Oct 12. Brain Struct Funct. 2010. PMID: 20938680 Free PMC article.
References
- Airaksinen MS, Koltzenburg M, Lewin GR, Masu Y, Helbig C, Wolf E, Brem G, Toyka KV, Thoenen H, Meyer M. Specific subtypes of cutaneous mechanoreceptors require neurotrophin-3 following peripheral target innervation. Neuron. 1996;16:287–295. - PubMed
- Barbacid M. The Trk family of neurotrophin receptors [review]. J Neurobiol. 1994;25:1386–1403. - PubMed
- Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons [review]. Prog Clin Biol Res. 1994;390:45–56. - PubMed
- Barker PA, Lomen-Hoerth C, Gensch EM, Meakin SO, Glass DJ, Shooter EM. Tissue-specific alternative splicing generates two isoforms of the trkA receptor. J Biol Chem. 1993;268:15150–15157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials