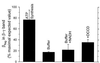Subunit rotation in Escherichia coli FoF1-ATP synthase during oxidative phosphorylation - PubMed (original) (raw)
Subunit rotation in Escherichia coli FoF1-ATP synthase during oxidative phosphorylation
Y Zhou et al. Proc Natl Acad Sci U S A. 1997.
Abstract
We report evidence for proton-driven subunit rotation in membrane-bound FoF1-ATP synthase during oxidative phosphorylation. A betaD380C/gammaC87 crosslinked hybrid F1 having epitope-tagged betaD380C subunits (betaflag) exclusively in the two noncrosslinked positions was bound to Fo in F1-depleted membranes. After reduction of the beta-gamma crosslink, a brief exposure to conditions for ATP synthesis followed by reoxidation resulted in a significant amount of betaflag appearing in the beta-gamma crosslinked product. Such a reorientation of gammaC87 relative to the three beta subunits can only occur through subunit rotation. Rotation was inhibited when proton transport through Fo was blocked or when ADP and Pi were omitted. These results establish FoF1 as the second example in nature where proton transport is coupled to subunit rotation.
Figures
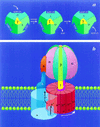
Figure 1
The binding change mechanism for FoF1 ATP synthases. This figure was adapted from ref. and modified. (a) Looking up at F1 from the membrane. In step 1, the asymmetric γ subunit rotates 120° clockwise driving conformational changes in the three catalytic sites that alter their affinities for substrates and product. In this illustration, the catalytic sites remain stationary. In step 2, ATP forms spontaneously from tightly bound ADP and Pi. For additional details and alternative views see refs. , , and . (b) View from the side of FoF1. The a-subunit contains two partial channels, each in contact with a different side of the membrane. In order for a H+ to traverse the membrane it moves through one channel to the center of the membrane, binds to one of the c-subunits (at Asp-61), and then is carried to the other partial channel by rotation of the c-subunit complex. The c-subunits are anchored to γ (11), whereas the a-subunit is anchored through subunits b and δ to the periphery of the α3β3 hexamer (12, 13). Hence the rotation of c-subunits relative to the a-subunit in Fo will drive the rotation of γ relative to the α3β3 hexamer in F1.
Figure 2
Rotation of subunits in E. coli FoF1 under ATP synthesis conditions. Hybrid F1 was prepared so that complexes containing a βD380C/γC87 crosslink contained βflagD380C subunits only in the two noncrosslinked β positions. After rebinding hybrid F1 to F1-depleted membranes, aliquots (1 mg total protein per ml) were exposed to different conditions (described below) for 30 sec at 23°C, 20 mM DTT and 2 mM selenocystamine were added to rapidly reduce any disulfide bonds, and the membranes were incubated for an additional 30 sec before passage through a Sephadex G50-F centrifuge column (30), equilibrated with TSGMg buffer. Disulfide bond formation was induced as each sample eluted from the column into a tube containing DTNB (0.2 mM final concentration). An aliquot of each oxidized sample (equivalent to 0.4 μg of βflag-F1) was denatured under nonreducing conditions and used for SDS/PAGE and immunoblotting. The blot above shows bands containing the βflagD380C subunit. As shown in lanes 1–4, membranes were exposed to the following conditions: lane 1, conditions for ATP synthesis (TSGMg buffer containing 4 mM ADP/20 mM Pi/2 mM NADH/165 units hexokinase/ml); lane 2, same as for lane 1 except that ADP, Pi, and NADH were omitted; lane 3, same as for lane 1 except that ADP and Pi were omitted; lane 4, same as for lane 1 except that F1-depleted membranes were pretreated with DCCD prior to reconstitution with hybrid F1 (see Materials and Methods). For the “noncrosslinked hybrid” control in lane 5, hybrid F1 was prepared from dissociated subunits without prior crosslinking of γC87 to a βD380C subunit. Thus, epitope-tagged β subunit could assemble randomly in the three β positions around the γC87 subunit. After rebinding to membranes and exposure to ATP synthesis conditions (as for lane 1), reoxidation of this sample provided a measure of the amount of βflagD380C trapped in the β–γ crosslinked product when the orientation of γC87 is random relative to the three β positions.
Figure 3
Quantitation of the Flag epitope appearing in the β–γ crosslinked product. For immunoblots as in Fig. 2, the amount of βflag in the 86-kDa band of each sample was determined by scanning densitometry. The value obtained for a “noncrosslinked hybrid” control (see Fig. 2, lane 5) was multiplied by 0.8 to correct for its greater βflag content compared with the crosslinked hybrid F1 (Fig. 2, lanes 1–4). This value was set to 100%, representing the amount of βflag expected in the β–γ crosslinked product if each β subunit has an equal opportunity to crosslink to γ following reduction and exposure to conditions for ATP synthesis. Bars are labeled to indicate conditions as described for Fig. 2. Data from three separate experiments were averaged and the error bars represent standard deviations.
Similar articles
- Rotation of the epsilon subunit during catalysis by Escherichia coli FOF1-ATP synthase.
Bulygin VV, Duncan TM, Cross RL. Bulygin VV, et al. J Biol Chem. 1998 Nov 27;273(48):31765-9. doi: 10.1074/jbc.273.48.31765. J Biol Chem. 1998. PMID: 9822640 - Rotation of subunits during catalysis by Escherichia coli F1-ATPase.
Duncan TM, Bulygin VV, Zhou Y, Hutcheon ML, Cross RL. Duncan TM, et al. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):10964-8. doi: 10.1073/pnas.92.24.10964. Proc Natl Acad Sci U S A. 1995. PMID: 7479919 Free PMC article. - Coupling H+ transport and ATP synthesis in F1F0-ATP synthases: glimpses of interacting parts in a dynamic molecular machine.
Fillingame RH. Fillingame RH. J Exp Biol. 1997 Jan;200(Pt 2):217-24. doi: 10.1242/jeb.200.2.217. J Exp Biol. 1997. PMID: 9050229 Review. - ATP hydrolysis by membrane-bound Escherichia coli F0F1 causes rotation of the gamma subunit relative to the beta subunits.
Zhou Y, Duncan TM, Bulygin VV, Hutcheon ML, Cross RL. Zhou Y, et al. Biochim Biophys Acta. 1996 Jul 18;1275(1-2):96-100. doi: 10.1016/0005-2728(96)00056-4. Biochim Biophys Acta. 1996. PMID: 8688454 Review. - Amino Acid Residues β139, β189, and β319 Modulate ADP-Inhibition in Escherichia coli H+-FOF1-ATP Synthase.
Lapashina AS, Shugaeva TE, Berezina KM, Kholina TD, Feniouk BA. Lapashina AS, et al. Biochemistry (Mosc). 2019 Apr;84(4):407-415. doi: 10.1134/S0006297919040084. Biochemistry (Mosc). 2019. PMID: 31228932
Cited by
- Conformational dynamics of the F1-ATPase beta-subunit: a molecular dynamics study.
Böckmann RA, Grubmüller H. Böckmann RA, et al. Biophys J. 2003 Sep;85(3):1482-91. doi: 10.1016/S0006-3495(03)74581-0. Biophys J. 2003. PMID: 12944266 Free PMC article. - A biological molecular motor, proton-translocating ATP synthase: multidisciplinary approach for a unique membrane enzyme.
Sambongi Y, Ueda I, Wada Y, Futai M. Sambongi Y, et al. J Bioenerg Biomembr. 2000 Oct;32(5):441-8. doi: 10.1023/a:1005656706248. J Bioenerg Biomembr. 2000. PMID: 15254379 - The regulatory switch of F1-ATPase studied by single-molecule FRET in the ABEL Trap.
Bockenhauer SD, Duncan TM, Moerner WE, Börsch M. Bockenhauer SD, et al. Proc SPIE Int Soc Opt Eng. 2014 Apr 1;8950:89500H. doi: 10.1117/12.2042688. Proc SPIE Int Soc Opt Eng. 2014. PMID: 25309100 Free PMC article. - Movements of the epsilon-subunit during catalysis and activation in single membrane-bound H(+)-ATP synthase.
Zimmermann B, Diez M, Zarrabi N, Gräber P, Börsch M. Zimmermann B, et al. EMBO J. 2005 Jun 15;24(12):2053-63. doi: 10.1038/sj.emboj.7600682. Epub 2005 May 26. EMBO J. 2005. PMID: 15920483 Free PMC article. - Precise packaging of the three genomic segments of the double-stranded-RNA bacteriophage phi6.
Mindich L. Mindich L. Microbiol Mol Biol Rev. 1999 Mar;63(1):149-60. doi: 10.1128/MMBR.63.1.149-160.1999. Microbiol Mol Biol Rev. 1999. PMID: 10066834 Free PMC article. Review.
References
- Capaldi R A, Aggeler R, Wilkens S, Gruber G. J Bioenerg Biomembr. 1996;28:397–401. - PubMed
- Cross R L, Duncan T M. J Bioenerg Biomembr. 1996;28:403–408. - PubMed
- Deckers-Hebestreit G, Altendorf K. Annu Rev Microbiol. 1996;50:791–824. - PubMed
- Howitt S M, Rodgers J W, Hatch L P, Gibson F, Cox G B. J Bioenerg Biomembr. 1996;28:415–420. - PubMed
- Nakamoto R K. J Membr Biol. 1996;151:101–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous