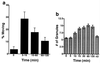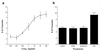Neurotrophin-3 signals redistribute RNA in neurons - PubMed (original) (raw)
Neurotrophin-3 signals redistribute RNA in neurons
R B Knowles et al. Proc Natl Acad Sci U S A. 1997.
Abstract
The translocation of specific mRNAs to dendrites and their potential for locally regulated translation are likely to serve as an effector in neuronal plasticity. Whether translation in dendrites is regulated by delivery of the RNA to sites of plasticity or a stationary pool of localized RNA undergoes enhanced translational efficiency is not clear. We show that RNA can translocate into dendrites in response to NT-3. RNA granules were visualized in cultured rat cortical neurons using the dye SYTO 14, which labels poly-ribosome complexes. Long before the morphological effects of NT-3 appeared, there was increased distal translocation of labeled complexes. This effect was blocked by K252a, a potent inhibitor of tyrosine kinase receptors. Therefore, neurons can utilize extracellular signals to alter the distribution of protein synthetic machinery via the active transport of RNA granules.
Figures
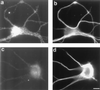
Figure 1
Immunofluorescence micrographs of 4-day-old cultured cortical neurons double-labeled with trkC and a neuron specific tubulin monoclonal antibody. (a) Positive neuronal staining with trkC antibody and (b) corresponding staining with the tubulin antibody. (c) A negatively stained neuron with trkC antibody and (d) corresponding staining with neuronal specific tubulin antibody. (Bar = 10 μm.)
Figure 2
Time lapse of RNA granule transport in neurites of cultured cortical neurons stimulated by NT-3 (a) SYTO 14-labeled neurites 5 min after NT-3 treatment in which arrowheads point to the baseline locations of those RNA granules present at the initiation of the experiment (colorized in red). The remaining labeled intracellular structures are mitochondria. The end of the proximal neuritic shaft is located at the bottom left hand corner. (b–i) Micrographs show neurites at 1 min intervals from 8 to 15 min after NT-3 treatment. Arrows point to some of those RNA granules that appeared during the interval (colorized in green). (c) New granules first appeared close to the proximal shaft at 9 min and (d–i) are observed to move distally into the neurites (arrows). (Bar = 10 μm.)
Figure 3
Rapid increase in RNA granule movement and density in neurites of cultured cortical neurons due to NT-3 treatment. (a) The percent of SYTO 14-labeled RNA granules that were observed moving over a 10 min period at varying times after NT-3 treatment (50 ng/ml) (n = 25). Between 5–15 min after adding NT-3 there was a 9-fold increase in the number of granules moving (P < 0.001). Between 15 and 60 min, the number of moving granules decreased, but was still 4-fold higher than baseline (P < 0.01). (b) Number of SYTO 14-labeled RNA granules per 40 μm of nonaxonal neurite of 4-day-old cortical cultures were measured at different time points after treatment with NT-3 (50 ng/ml). Time 10 min is significantly different from time 0 (P < 0.01); F test demonstrated inequality of variances for control vs. time points 15 through 120 min (P < 0.05) and Satterwaite approximation for samples of unequal variances demonstrated means significantly different for these time points (P < 0.001). Control lane N2 only received N2 supplements for 2 h. (n = 50 for all lanes).
Figure 4
Increase in RNA granule density due to NT-3 treatment is dose dependent and blocked by a trk inhibitor. (a) Number of SYTO 14-labeled RNA granules per 40 μm of nonaxonal neurite in 4-day-old cortical cultures after 1 h of treatment with varying concentrations of NT-3 (n = 50); (b) Similar measurements in cells treated with 0.01% dimethyl sulfoxide (control), 1 h of 30 nM K252a; 1 h of pretreatment with 30 nM K252a then 1 h of 30 nM K252a and 50 ng/ml NT-3; and 1 h of 50 ng/ml NT-3 (n = 50 all lanes). Treatment with K252a blocked the effect of NT-3 (P < 0.001).
Similar articles
- Neuronal RNA localization and the cytoskeleton.
Bassell GJ, Singer RH. Bassell GJ, et al. Results Probl Cell Differ. 2001;34:41-56. doi: 10.1007/978-3-540-40025-7_3. Results Probl Cell Differ. 2001. PMID: 11288678 Review. No abstract available. - A molecular mechanism for mRNA trafficking in neuronal dendrites.
Shan J, Munro TP, Barbarese E, Carson JH, Smith R. Shan J, et al. J Neurosci. 2003 Oct 1;23(26):8859-66. doi: 10.1523/JNEUROSCI.23-26-08859.2003. J Neurosci. 2003. PMID: 14523087 Free PMC article. - RNA granule protein 140 (RNG140), a paralog of RNG105 localized to distinct RNA granules in neuronal dendrites in the adult vertebrate brain.
Shiina N, Tokunaga M. Shiina N, et al. J Biol Chem. 2010 Jul 30;285(31):24260-9. doi: 10.1074/jbc.M110.108944. Epub 2010 Jun 1. J Biol Chem. 2010. PMID: 20516077 Free PMC article. - Prevention of apoptotic but not necrotic cell death following neuronal injury by neurotrophins signaling through the tyrosine kinase receptor.
Kim DH, Zhao X, Tu CH, Casaccia-Bonnefil P, Chao MV. Kim DH, et al. J Neurosurg. 2004 Jan;100(1):79-87. doi: 10.3171/jns.2004.100.1.0079. J Neurosurg. 2004. PMID: 14743916 - Perplexing bodies: The putative roles of P-bodies in neurons.
Zeitelhofer M, Macchi P, Dahm R. Zeitelhofer M, et al. RNA Biol. 2008 Oct-Dec;5(4):244-8. doi: 10.4161/rna.6948. Epub 2008 Oct 8. RNA Biol. 2008. PMID: 19182533 Review.
Cited by
- Profiling axonal mRNA transport.
Willis DE, Twiss JL. Willis DE, et al. Methods Mol Biol. 2011;714:335-52. doi: 10.1007/978-1-61779-005-8_21. Methods Mol Biol. 2011. PMID: 21431751 Free PMC article. - A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons.
Zheng JQ, Kelly TK, Chang B, Ryazantsev S, Rajasekaran AK, Martin KC, Twiss JL. Zheng JQ, et al. J Neurosci. 2001 Dec 1;21(23):9291-303. doi: 10.1523/JNEUROSCI.21-23-09291.2001. J Neurosci. 2001. PMID: 11717363 Free PMC article. - Dendritically localized transcripts are sorted into distinct ribonucleoprotein particles that display fast directional motility along dendrites of hippocampal neurons.
Tübing F, Vendra G, Mikl M, Macchi P, Thomas S, Kiebler MA. Tübing F, et al. J Neurosci. 2010 Mar 17;30(11):4160-70. doi: 10.1523/JNEUROSCI.3537-09.2010. J Neurosci. 2010. PMID: 20237286 Free PMC article. - Expression and branch-specific export of mRNA are regulated by synapse formation and interaction with specific postsynaptic targets.
Schacher S, Wu F, Panyko JD, Sun ZY, Wang D. Schacher S, et al. J Neurosci. 1999 Aug 1;19(15):6338-47. doi: 10.1523/JNEUROSCI.19-15-06338.1999. J Neurosci. 1999. PMID: 10414963 Free PMC article.
References
- Thoenen H. Science. 1995;270:593–598. - PubMed
- Krug M, Lossner B, Ott T. Brain Res Bull. 1984;13:39–42. - PubMed
- Steward O. Curr Opin Neurobiol. 1995;5:55–61. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials