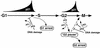Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition - PubMed (original) (raw)
Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition
V Dulić et al. Mol Cell Biol. 1998 Jan.
Abstract
Cell cycle arrest in G1 in response to ionizing radiation or senescence is believed to be provoked by inactivation of G1 cyclin-cyclin-dependent kinases (Cdks) by the Cdk inhibitor p21(Cip1/Waf1/Sdi1). We provide evidence that in addition to exerting negative control of the G1/S phase transition, p21 may play a role at the onset of mitosis. In nontransformed fibroblasts, p21 transiently reaccumulates in the nucleus near the G2/M-phase boundary, concomitant with cyclin B1 nuclear translocation, and associates with a fraction of cyclin A-Cdk and cyclin B1-Cdk complexes. Premitotic nuclear accumulation of cyclin B1 is not detectable in cells with low p21 levels, such as fibroblasts expressing the viral human papillomavirus type 16 E6 oncoprotein, which functionally inactivates p53, or in tumor-derived cells. Moreover, synchronized E6-expressing fibroblasts show accelerated entry into mitosis compared to wild-type cells and exhibit higher cyclin A- and cyclin B1-associated kinase activities. Finally, primary embryonic fibroblasts derived from p21-/- mice have significantly reduced numbers of premitotic cells with nuclear cyclin B1. These data suggest that p21 promotes a transient pause late in G2 that may contribute to the implementation of late cell cycle checkpoint controls.
Figures
FIG. 1
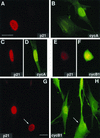
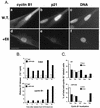
Nuclear colocalization of p21 with cyclin A and cyclin B1 in exponentially growing normal human fibroblasts. Asynchronous normal HDF (Hs68) were fixed in paraformaldehyde and simultaneously stained with mouse monoclonal anti-p21 (red; Texas red) and with rabbit polyclonal anti-cyclin A or anti-cyclin B1 (green; fluorescein) antibodies as described in Materials and Methods. Representative micrographs of cells in G1 phase (A and B), S phase (A, B, G, and H), and G2 phase (C, D, G, and H), and mitosis (E and F) are shown. Cyclin A accumulates in the nucleus in the beginning of the S phase, whereas cyclin B1 accumulates during late S phase and in G2 in the cytoplasm and enters the nucleus at the onset of mitosis (27). A cell with both cytoplasmic and nuclear cyclin B1 accumulation is marked with an arrow (G and H). Quantitation of localization experiments is shown in Tables 1 and 2. Exposure times for given antigen were constant for all micrographs. Bars, 10 μm.
FIG. 2
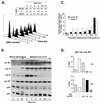
Reaccumulation of p21 at G2/M-phase boundary in synchronized normal human fibroblasts. (A) FACS analysis of synchronized Hs68 fibroblasts at different times after release from an aphidicolin block. The percentage of cells in different phases of the cell cycle was determined by using the CellFit program (Materials and Methods). (B) Immunoblot analysis of cell extracts. Fibroblasts were synchronized either in G0 by serum starvation for 72 h, followed by serum stimulation for 9 and 24 h, or at the G1/S boundary, by a combination of serum stimulation (12 h) and aphidicolin block (20 h). Total-cell extracts were prepared from cells at the indicated times after serum stimulation or release from the block, analyzed by SDS-PAGE (8.5% gel for cyclins [cyc] B1, A, E, and D1; 12% gel for p27 and p21), and immunoblotted with specific antibodies against cyclins and CKIs. (C) Cyclin A- and cyclin B1-associated histone H1 kinase activities. Kinase activity of the cyclin A and cyclin B1 complexes immunoprecipitated from cell extracts described above was tested by using histone H1 as the substrate as described in Materials and Methods. Cells in late G1 (quiescent cells serum stimulated for 15 h) were used as a negative control. (D) Colocalization of p21 and cyclin B1 in cells synchronized by aphidicolin block at the G1/S boundary. Total populations of arrested cells (0 h [G1/S boundary]) and the cells after a release from the block (10 h [G2/M boundary]) were analyzed as described for Table 2. At least 500 cells were scored for each time point. cyt, cytoplasmic; cn, cytoplasmic and/or nuclear.
FIG. 3
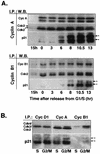
Increasing association of p21 with cyclin A and cyclin B1 in G2/M-phase cells. (A) Western blot analysis of cyclin complexes from lysates of synchronized Hs68 cells. Cyclin (Cyc) A and cyclin B1 complexes were immunoprecipitated (I.P.) from total lysates prepared from cells stimulated with serum for 15 h and cells released from aphidicolin block at the indicated time points. Immune complexes were separated on SDS–12% polyacrylamide gels, transferred to an Immobilon membrane, and detected by using the indicated antibodies (W.B. [Western blotting]) by ECL. Note that cyclin B1 immunoblots had to be exposed much longer than cyclin A immunoblots. (B) Comparative analysis of cyclin D1, cyclin A, and cyclin B1 immunocomplexes isolated from S-phase (pooled 0-, 3-, and 6-h time points)- and G2/M-phase (pooled 10.5- and 13-h time points)-enriched cell lysates. The resulting immunocomplexes were resolved on the same SDS–12% polyacrylamide gel. Immunoblots were probed with either Cdk-specific antibodies (anti-Cdk4, anti-PSTAIRE for Cdk2 and Cdc2) or anti-p21, as indicated. Arrows with asterisks indicate differently phosphorylated species of p21.
FIG. 4
p21-associated cyclin (cyc)-Cdk2 complexes are inactive. Extracts prepared from normal fibroblasts (Hs68) synchronized in G1, S, and G2/M phases are depleted of p21. (A) Western blot analysis of p21 immunoprecipitates (IP) from the different cell extracts (200 μg) and total proteins in the corresponding lysates (40 μg). (B) Western blot analysis of total proteins in cell extracts (40 μg) depleted (+ α-p21) or not depleted (− α-p21) of p21. (C) Western blot analysis of cyclin A and cyclin B1 immunocomplexes isolated from the p21-depleted and mock-depleted extracts (150 μg). (D) Cyclin A- or B1-associated histone H1 kinase activity. In these experiments, cyclin A and cyclin B1 immunocomplexes, assayed for kinase activity by using histone H1 as a substrate, were separated by SDS-PAGE (11% gel), transferred onto an Immobilon membrane and simultaneously analyzed for the presence of cyclins and Cdks by Western blotting, and exposed to reveal histone H1-associated radioactivity. In addition, Coomassie blue-stained histone H1 bands remaining on the gel (about 50%) were excised, and associated radioactivity was analyzed by Cerenkov counting. The immunoblots were probed with indicated antibodies, except that in cyclin B1 immunoprecipitates, Cdc2 was also detected by using an anti-PSTAIRE monoclonal antibody. The extent of removal of cyclins or Cdks upon p21 depletion was evaluated by densitometric scanning of immunoblots. Note that in G2/M cells, cyclin A increasingly associates with unphosphorylated Cdk2 (indicated by an arrow in panel C). Arrows with asterisks in panels A and B indicate phosphorylated Cdk species.
FIG. 5
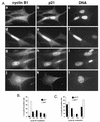
Deregulated G2/M-phase transition in p53-deficient cells. (A) Nuclear localization of cyclin B1 in wild-type cells (W.T.; IMR-90) and fibroblasts expressing low levels of p21 owing to expression of HPV16 E6 (+E6) as described in Materials and Methods. Formalin-fixed cells were stained with cyclin B1- (fluorescein; a and d) and p21Cip1-specific (Texas red; b and e) antibodies. Nuclei were counterstained with Hoechst 33258 to assess the state of DNA condensation (c and f). Experimental conditions were the same as those described for Fig. 1. Note the absence of nucleoli and the signs of DNA condensation in E6 cells accumulating nuclear cyclin B1. Bar, 10 μm. (B) Cyclin B1- and Cdk2-associated histone H1 kinase activity from aliquots prepared from synchronized wild-type and E6 fibroblasts. Cells were synchronized at the G1/S-phase boundary by aphidicolin block as described in the legend to Fig. 2. (C) Accelerated entry into mitosis of p53− p21− cells. The late stages of the cell cycle in synchronized wild-type and E6 cultures were analyzed based on subcellular distribution of cyclin B1. Cells were released from G1/S-phase block (hydroxyurea) for 6 and 10 h as described in Materials and Methods. Note that only cells accumulating cytoplasmic and nuclear cyclin B1 were scored. The following cyclin B1-specific staining patterns were distinguished: Cyt, cytoplasmic (like in Fig. 1H); CN, cytoplasmic and nuclear, no visible nucleoli (like in Fig. 1H); CN*, cytoplasmic and nuclear, with visible nucleoli (like in Fig. 1H); Nuc, predominantly nuclear with visible nucleoli (like in Fig. 1H and in 3A); PM, prophase and metaphase (like in Fig. 1F).
FIG. 6
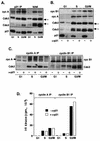
p21−/− MEFs do not accumulate nuclear cyclin B1 before mitosis. (A) Nuclear colocalization of cyclin B1 and p21 in wild-type (a to i) and p21−/− (j to l) MEFs. The nuclei were counterstained with Hoechst 33258 (DNA) to assess the state of DNA condensation. Representative cells exhibiting cytoplasmic and nuclear (a) and nuclear (d and g) cyclin B1 localization are shown. Micrograph I shows a p21−/− cell with nuclear cyclin B1 with ongoing DNA condensation. (B) Quantitative analysis of p21-cyclin B1 colocalization in exponentially growing MEFs. Note that only cyclin B1-positive cells were scored. We distinguished cells with apparent accumulation of cyclin B1 in both in the cytoplasm and the nucleus without (CN) and with (CN*) visible nucleoli, predominantly in the nucleus (Nuc) as well as those in different stages of prophase (Pro). (C) Quantitative analysis showing cyclin B1 localization in wild-type (W.T.) and p21−/− MEFs. Only cells showing nuclear signal were scored.
FIG. 7
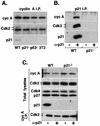
p21 association with cyclin A-Cdk2 complexes in MEFs. (A) Immunoprecipitates (I.P.) of cyclin (cyc) A immunocomplexes were isolated from extracts prepared from exponentially growing p21+ (wild-type p21+/+ [WT]; MEF and NIH 3T3) and p21− (p21−/− and p53−/− MEF) cells and were analyzed by immunoblotting for the presence of Cdk2 and p21Cip1. All fibroblasts were at passage 4. (B and C) Depletion of p21 in MEF extracts. Whole-cell extracts prepared from wild-type and p21−/− MEFs were incubated with p21-specific antibodies (+ α-p21) or protein A-Sepharose beads (− α-p21). (B) Western blot analysis of p21 immunoprecipitates tested for the presence of cyclin A and Cdk2. (C) Immunoblot analysis of aliquots of the p21-depleted or mock-depleted extracts for the presence of cyclin A, Cdk2, Cdk4, p27Kip1, and p21. The lower part of panel C shows immunoblot analysis (probed with anti-Cdk2) of cyclin A immunoprecipitates prepared from the same p21-depleted extracts. Arrows indicate two forms of Cdk2; the lower form represents Thr160-phosphorylated Cdk2.
FIG. 8
Model: p21 involvement in G2/M checkpoint control? In nontransformed (p53+) cells, nuclear accumulation of p21 and binding to cyclin-Cdk complexes during G1 and before mitosis (late G2) may facilitate checkpoint implementation at the G1/S-phase and G2/M-phase transitions. In G2, a p21-induced pause might potentiate the integration of G2 checkpoint signals that regulate entry into mitosis through modulating the activity of cyclin A-Cdk2 and cyclin B1-Cdc2 complexes. Alternatively, increasing association of p21 with Cdk complexes at both transition points may sensitize these kinases to respond to further increases of p21 resulting from DNA damage. Whereas it seems that p53 activity (and p21 accumulation) is required for DNA damage-induced G1 arrest, p53 (and p21) may be only a part of a redundant mechanism regulating G2 arrest.
Similar articles
- Delta MEKK3:ER* activation induces a p38 alpha/beta 2-dependent cell cycle arrest at the G2 checkpoint.
Garner AP, Weston CR, Todd DE, Balmanno K, Cook SJ. Garner AP, et al. Oncogene. 2002 Nov 21;21(53):8089-104. doi: 10.1038/sj.onc.1206000. Oncogene. 2002. PMID: 12444545 - Transient suppression of nuclear Cdc2 activity in response to ionizing radiation.
Kim MJ, Lee JY, Lee SJ. Kim MJ, et al. Oncol Rep. 2008 May;19(5):1323-9. Oncol Rep. 2008. PMID: 18425394 - Deregulation of p53/p21Cip1/Waf1 pathway contributes to polyploidy and apoptosis of E1A+cHa-ras transformed cells after gamma-irradiation.
Bulavin DV, Tararova ND, Aksenov ND, Pospelov VA, Pospelova TV. Bulavin DV, et al. Oncogene. 1999 Oct 7;18(41):5611-9. doi: 10.1038/sj.onc.1202945. Oncogene. 1999. PMID: 10523840 - Regulation of the G2/M transition by p53.
Taylor WR, Stark GR. Taylor WR, et al. Oncogene. 2001 Apr 5;20(15):1803-15. doi: 10.1038/sj.onc.1204252. Oncogene. 2001. PMID: 11313928 Review. - [Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].
Viallard JF, Lacombe F, Belloc F, Pellegrin JL, Reiffers J. Viallard JF, et al. Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
Cited by
- p21WAF1 expression in invasive breast cancer and its association with p53, AP-2, cell proliferation, and prognosis.
Pellikainen MJ, Pekola TT, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Pellikainen MJ, et al. J Clin Pathol. 2003 Mar;56(3):214-20. doi: 10.1136/jcp.56.3.214. J Clin Pathol. 2003. PMID: 12610102 Free PMC article. - CD4+CD25+ T regulatory cells from FIV+ cats induce a unique anergic profile in CD8+ lymphocyte targets.
Fogle JE, Tompkins WA, Tompkins MB. Fogle JE, et al. Retrovirology. 2010 Nov 19;7:97. doi: 10.1186/1742-4690-7-97. Retrovirology. 2010. PMID: 21092106 Free PMC article. - Activity and nature of p21(WAF1) complexes during the cell cycle.
Cai K, Dynlacht BD. Cai K, et al. Proc Natl Acad Sci U S A. 1998 Oct 13;95(21):12254-9. doi: 10.1073/pnas.95.21.12254. Proc Natl Acad Sci U S A. 1998. PMID: 9770473 Free PMC article. - Complete inhibition of Cdk/cyclin by one molecule of p21(Cip1).
Hengst L, Göpfert U, Lashuel HA, Reed SI. Hengst L, et al. Genes Dev. 1998 Dec 15;12(24):3882-8. doi: 10.1101/gad.12.24.3882. Genes Dev. 1998. PMID: 9869641 Free PMC article. - Mutant MyoD lacking Cdc2 phosphorylation sites delays M-phase entry.
Tintignac LA, Sirri V, Leibovitch MP, Lécluse Y, Castedo M, Metivier D, Kroemer G, Leibovitch SA. Tintignac LA, et al. Mol Cell Biol. 2004 Feb;24(4):1809-21. doi: 10.1128/MCB.24.4.1809-1821.2004. Mol Cell Biol. 2004. PMID: 14749395 Free PMC article.
References
- Aprelikova O, Xiong Y, Liu E T. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J Biol Chem. 1995;270:18195–18197. - PubMed
- Bailly E, Pines J, Hunter T, Bornens M. Cytoplasmic accumulation of cyclin B1 in human cells: association with a detergent-resistant compartment and with the centrosome. J Cell Sci. 1992;101:529–545. - PubMed
- Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous