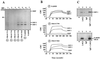The paired Ig-like receptor PIR-B is an inhibitory receptor that recruits the protein-tyrosine phosphatase SHP-1 - PubMed (original) (raw)
The paired Ig-like receptor PIR-B is an inhibitory receptor that recruits the protein-tyrosine phosphatase SHP-1
M Bléry et al. Proc Natl Acad Sci U S A. 1998.
Abstract
An emerging family of cell surface inhibitory receptors is characterized by the presence of intracytoplasmic immunoreceptor tyrosine-based inhibition motifs (ITIM). These ITIM-bearing inhibitory receptors, which are typically paired with activating isoforms, associate with Src homology domain 2-containing phosphatases following ITIM tyrosine phosphorylation. Two categories of phosphatases are recruited by the ITIM-bearing receptors: the protein-tyrosine phosphatases, SHP-1 and SHP-2, and the polyphosphate inositol 5-phosphatase, SHIP. The dynamic equilibrium of B cell activation is partially controlled by two well known ITIM-bearing receptors, CD22 and FcgammaRIIB, a low affinity receptor for IgG. We describe here that a murine ITIM-bearing molecule, PIR-B, can also negatively regulate B cell activation. Tyrosine-phosphorylated ITIMs allow PIR-B to associate with SHP-1 but not with SHIP. Engagement of PIR-B thereby initiates a SHP-1-dependent inhibitory pathway that may play an important role in regulating B lymphocyte activation.
Figures
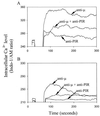
Figure 1
Inhibition of BCR-induced Ca2+ mobilization by coligation with PIR molecules. Mouse splenic B cells loaded with INDO-1 dye were analyzed by flow cytometry for intracellular Ca2+ levels in the presence (A) or absence (B) of extracellular Ca2+. Cells were stimulated with biotinylated F(ab′)2 fragments of anti-μ and anti-PIR mAbs either alone or in combination as indicated by arrows. Biotin (10 μg) was used as a cross-linker. Independent ligation with the anti-PIR mAb had no inhibitory effect on the anti-μ induced Ca2+ response (not shown).
Figure 2
Expression of FcγRIIB/PIR-B chimeric molecules by transfected rat basophil leukemia (RBL) cells. (A) Schematic representation of chimeric molecules. The FcγRIIB1 sequence is indicated in light gray, and the PIR-B sequences in dark gray. Numbers over the bars indicate amino acids included in each region of the protein (EC, extracellular; TM, transmembrane; and IC, intracellular). Tyrosines in the cytoplasmic regions of the chimeric molecules are numbered according to the wild-type PIR-B sequence. Tyrosine (Y) to phenylalanine (F) mutations are indicated for each point mutant construction. (B) Cell surface expression of chimeric molecules by RBL transfectants was assayed by indirect immunofluorescence staining with the 2.4G2 anti-FcγRII/III mAb (filled profile). FcɛRI expression was revealed by staining with the BC4 anti-FcɛR mAb (thin line), and irrelevant isotype-matched mAbs served as negative controls (bold lines). Flow cytometric analysis of the FcγRIIB/PIR-B.L (2A10, Upper) and FcγRIIB/PIR-B.H (2F7, Lower) clones indicates expression of the FcγRIIB/PIR-B chimeric molecule at relatively low and high densities, respectively.
Figure 3
Inhibition of FcɛRI-dependent cell activation by co-engagement of FcγRIIB/PIR-B chimeric molecules. (A) Assay of serotinin release by FcγRIIB/PIR-B.H cells (•) and mock transfectant cells (○). Cells were stimulated with varying dilutions of mouse IgE-producing hybridoma supernatants and the 2.4G2 anti-FcγRII/III mAb (20 μg/ml). Wild-type RBL cells yielded similar results to the mock RBL transfectant (not shown). Results expressed as mean ± SD of three independent experiments. (B) Analysis of serotonin release after co-aggregation (striped bars) versus independent aggregation (black bars) of FcγRIIB/PIR-B chimeric molecules and FcɛRI. Co-aggregation was induced by incubation of the cells with the 2.4G2 anti-FcγRII/III mAb (20 μg/ml), mouse IgE (10−3 dilution), and F(ab′)2 GAM (50 μg/ml). Independent aggregation was induced by incubation of the cells with the 2.4G2 mAb (20 μg/ml) plus F(ab′)2 DAM (100 μg/ml) and rat IgE plus F(ab′)2 DAR (100 μg/ml). Percentage inhibition was defined as the serotonin release (in cpm) induced by mouse IgE stimulation and 2.4G2 minus the background release divided by the serotonin stimulation induced by mouse IgE stimulation minus the background release. Results are representative of three independent experiments. (C) Comparative analysis of the inhibitory functions of FcγRIIB/PIR-B mutants versus wild-type chimeras. Inhibition percentages were calculated according to the above formula. FcγRIIB/PIR-B.H, FcγRIIB/PIR-B.Y794F, and FcγRIIB/PIR-B.Y824F transfected RBL cells expressed comparative levels of chimeric molecule; FACS analysis indicated mean fluorescence intensities for 2.4G2 anti-FcγR staining of 138, 165, and 89 for FcγRIIB/PIR-B.H, FcγRIIB/PIR-B.Y794F, and FcγRIIB/PIR-B.Y824F, respectively. FcγRIIB/PIR-B.L and FcγRIIB/PIR-BY794F,Y824F have similar but lower 2.4G2 fluorescence staining intensities, being 59 and 44, respectively, for FcγRIIB/PIR-B.L and FcγRIIB/PIR-B.Y794F,Y824F. The difference in inhibition exerted by FcγRIIB/PIR-B versus the double mutant was confirmed in two additional experiments (P < 0.01).
Figure 4
Analysis of phosphatase binding of candidate ITIMs in the PIR-B cytoplasmic domain (A) PIR-B tyrosine-containing peptides were incubated with lysates prepared from metabolically 35S-labeled IIA1.6 B cells, and adsorbed molecules were separated under reducing conditions on an 8% SDS-PAGE. (B) Binding potential of phosphorylated PIR-B peptides to the GST-SHP 1.SH2(NC), GST-SHP 2.SH2(NC), and GST-SHIP.SH2 GST fusion proteins (150 nM) was analyzed with a BIAcore apparatus. The flow rate was constant at 10 μl/min. In this experiment, 80 resonance units of phosphorylated peptides were immobilized on streptavidin-coated sensorchips. The regeneration was performed using HBS buffer supplemented with 0.02% SDS. Results expressed as corrected resonance units (CRU) corresponding to raw values after subtraction of background RU values due to the injection medium. (C) Analysis of tyrosine phosphorylation induced by co-aggregation and independent aggregation of FcɛRI and the FcγRIIB/PIR-B chimera. Cell lysates of transfected RBL cells untreated (lane 1) or treated with the 2.4G2 anti-FcγR mAb (lane 2), mouse IgE (lane 3), or both 2.4G2 and mouse IgE (lane 4) followed by F(ab′)2 GAM were subjected to immunoprecipitation with protein-G beads. The bound materials were either separated on an SDS-8% PAGE, transferred onto membranes, and immunoblotted with anti-SHP-1 (Upper, 45 × 106 FcγRIIB/PIR-B.H cells per lane) or separated on an SDS-10% PAGE, transferred onto nitrocellulose membranes, and immunobotted with anti-phosphotyrosine antibodies (Lower, 5 × 106 FcγRIIB/PIR-B.H cells per lane). The experiment shown is representative of four independent experiments.
Similar articles
- SHP-1- and phosphotyrosine-independent inhibitory signaling by a killer cell Ig-like receptor cytoplasmic domain in human NK cells.
Yusa S, Catina TL, Campbell KS. Yusa S, et al. J Immunol. 2002 May 15;168(10):5047-57. doi: 10.4049/jimmunol.168.10.5047. J Immunol. 2002. PMID: 11994457 - Mast cell regulation via paired immunoglobulin-like receptor PIR-B.
Chen CC, Kong DW, Cooper MD, Kubagawa H. Chen CC, et al. Immunol Res. 2002;26(1-3):191-7. doi: 10.1385/ir:26:1-3:191. Immunol Res. 2002. PMID: 12403357 Review. - Cytoplasmic protein tyrosine phosphatases SHP-1 and SHP-2: regulators of B cell signal transduction.
Tamir I, Dal Porto JM, Cambier JC. Tamir I, et al. Curr Opin Immunol. 2000 Jun;12(3):307-15. doi: 10.1016/s0952-7915(00)00092-3. Curr Opin Immunol. 2000. PMID: 10781410 Review.
Cited by
- Is the CD200/CD200 receptor interaction more than just a myeloid cell inhibitory signal?
Minas K, Liversidge J. Minas K, et al. Crit Rev Immunol. 2006;26(3):213-30. doi: 10.1615/critrevimmunol.v26.i3.20. Crit Rev Immunol. 2006. PMID: 16928187 Free PMC article. Review. - Fc receptor-like 5 inhibits B cell activation via SHP-1 tyrosine phosphatase recruitment.
Haga CL, Ehrhardt GR, Boohaker RJ, Davis RS, Cooper MD. Haga CL, et al. Proc Natl Acad Sci U S A. 2007 Jun 5;104(23):9770-5. doi: 10.1073/pnas.0703354104. Epub 2007 May 23. Proc Natl Acad Sci U S A. 2007. PMID: 17522256 Free PMC article. - Paired immunoglobulin-like receptor A is an intrinsic, self-limiting suppressor of IL-5-induced eosinophil development.
Ben Baruch-Morgenstern N, Shik D, Moshkovits I, Itan M, Karo-Atar D, Bouffi C, Fulkerson P, Rashkovan D, Jung S, Rothenberg ME, Munitz A. Ben Baruch-Morgenstern N, et al. Nat Immunol. 2014 Jan;15(1):36-44. doi: 10.1038/ni.2757. Epub 2013 Nov 10. Nat Immunol. 2014. PMID: 24212998 Free PMC article. - Paternal monoallelic expression of the paired immunoglobulin-like receptors PIR-A and PIR-B.
Chen CC, Hurez V, Brockenbrough JS, Kubagawa H, Cooper MD. Chen CC, et al. Proc Natl Acad Sci U S A. 1999 Jun 8;96(12):6868-72. doi: 10.1073/pnas.96.12.6868. Proc Natl Acad Sci U S A. 1999. PMID: 10359805 Free PMC article. - Phosphatase regulation of immunoreceptor signaling in T cells, B cells and mast cells.
Bounab Y, Getahun A, Cambier JC, Daëron M. Bounab Y, et al. Curr Opin Immunol. 2013 Jun;25(3):313-20. doi: 10.1016/j.coi.2013.04.001. Epub 2013 May 15. Curr Opin Immunol. 2013. PMID: 23684445 Free PMC article. Review.
References
- Vivier E, Daëron M. Immunol Today. 1997;18:286–291. - PubMed
- Burshtyn D N, Yang W, Yi T, Long E O. J Biol Chem. 1997;272:13066–13072. - PubMed
- Scharenberg A M, Kinet J-P. Cell. 1996;87:961–964. - PubMed
- Alley, T. L., Cooper, M. D., Chen, M. & Kubagawa, H. (1998) Tissue Antigens 51, in press. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous