ARF is required for maintenance of yeast Golgi and endosome structure and function - PubMed (original) (raw)
ARF is required for maintenance of yeast Golgi and endosome structure and function
E C Gaynor et al. Mol Biol Cell. 1998 Mar.
Free PMC article
Abstract
ADP ribosylation factor (ARF) is thought to play a critical role in recruiting coatomer (COPI) to Golgi membranes to drive transport vesicle budding. Yeast strains harboring mutant COPI proteins exhibit defects in retrograde Golgi to endoplasmic reticulum protein transport and striking cargo-selective defects in anterograde endoplasmic reticulum to Golgi protein transport. To determine whether arf mutants exhibit similar phenotypes, the anterograde transport kinetics of multiple cargo proteins were examined in arf mutant cells, and, surprisingly, both COPI-dependent and COPI-independent cargo proteins exhibited comparable defects. Retrograde dilysine-mediated transport also appeared to be inefficient in the arf mutants, and coatomer mutants with no detectable anterograde transport defect exhibited a synthetic growth defect when combined with arf1Delta, supporting a role for ARF in retrograde transport. Remarkably, we found that early and medial Golgi glycosyltransferases localized to abnormally large ring-shaped structures. The endocytic marker FM4-64 also stained similar, but generally larger ring-shaped structures en route from the plasma membrane to the vacuole in arf mutants. Brefeldin A similarly perturbed endosome morphology and also inhibited transport of FM4-64 from endosomal structures to the vacuole. Electron microscopy of arf mutant cells revealed the presence of what appear to be hollow spheres of interconnected membrane tubules which likely correspond to the fluorescent ring structures. Together, these observations indicate that organelle morphology is significantly more affected than transport in the arf mutants, suggesting a fundamental role for ARF in regulating membrane dynamics. Possible mechanisms for producing this dramatic morphological change in intracellular organelles and its relation to the function of ARF in coat assembly are discussed.
Figures
Figure 1
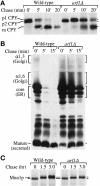
Anterograde protein transport kinetics and modification in wild-type and arf1Δ cells. (A) Wild-type (PSY315) and arf1Δ (TT104) cells were labeled with 35S-amino acids for 5 min and chased for 20 min at 30°C. Equal aliquots of cells were removed at the times indicated and the chase was terminated by addition of trichloroacetic acid (TCA). CPY was recovered from the samples by immunoprecipitation and subjected to SDS-PAGE. The positions of the ER (p1), Golgi (p2), and vacuolar (m) CPY forms are indicated. Similar results were obtained using the isogenic strains SEY6210 (wild-type) and 6210 arf1Δ, which were used for the remainder of the pulse-chase experiments. (B) Cells were labeled for 10 min at 20°C and chased for 15 min. The chase was terminated at the times indicated, and α-factor was recovered by immunoprecipitation and subjected to SDS-PAGE. The position of ER (core), early Golgi (α1,6), medial Golgi (α1,3), and late Golgi-secreted (mature) α-factor forms are indicated. (C) Cells were labeled for 15 min and chased for 3 h at 30°C. The chase was terminated at the times indicated and Mnn1p was recovered by immunoprecipitation and subjected to SDS-PAGE.
Figure 2
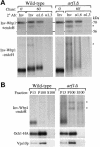
Posttranslational modification and localization of a KKXX-bearing Inv-Wbp1 fusion protein in wild-type and arf1Δ cells. Wild-type (SEY6210) and arf1Δ (6210 arf1Δ) cells harboring pEG1-KK were labeled for 10 min and chased for 60 min at 30°C. (A) The chase was terminated at the times indicated by the addition of TCA, and Inv-Wbp1p was recovered from the samples by immunoprecipitation with anti-invertase serum. The primary immunoprecipitates were boiled in 1% SDS to dissociate the antibody–antigen complex, and the eluates were split into three equal aliquots that were subjected to a second immunoprecipitation with antiserum to either invertase (Inv), α1,6-linked mannose (α1,6) or α1,3-linked mannose (α1,3). Half of each immunoprecipitate was subsequently treated with endoglycosidase H (+endo H) to examine proteolytic processing of the polypeptide, and half was left untreated (−endo H) to examine the effect of glycosylation on the fusion protein’s mobility in SDS gels. At the 0-min chase point, none of the Inv-Wbp1p was precipitated with the linkage-specific serum; therefore, only the anti-invertase immunoprecipitate is shown. The position of the intact fusion protein (70 kDa) and vacuolar processed (56 kDa) forms are shown. Asterisks indicate nonspecific bands contaminating the immunoprecipitates. (B) Labeled spheroplasts harboring pEG1-KK or pOH were harvested after 60 min of chase, lysed, and subjected to differential centrifugation as described previously (Gaynor et al., 1994). Inv-Wbp1p and the Golgi proteins Och1-HA and Vps10p were recovered from the 13,000 × g pellets (P13), 100,000 × g pellets (P100), and 100,000 × g supernatants (S100) by immunoprecipitation.
Figure 3
Synthetic defects in COPI/arf1Δ double mutants. (A) Growth of strains harboring the arf1Δ allele in combination with sec21–1 (γ-COP), sec21–2 (γ-COP), ret1–1 (α-COP), or sec27–1 (β′-COP) alleles on YPD plates was compared with the corresponding single mutants at the temperatures indicated. Complete genotypes of strains are provided in Table 1. +++, wild-type growth; ++, growth slower than wild type; +, very slow growth (minimal single colony formation); +/−, almost no growth (no single colony formation); and −, complete lethality. (B) COPI mutant strains harboring either the ARF1 wild-type (+) or arf1Δ (Δ) allele were labeled for 10 min and chased for 60 min at the temperature indicated. CPY was recovered by immunoprecipitation and subjected to SDS-PAGE. Positions of the ER (p1) and vacuolar (m) forms of CPY are indicated.
Figure 4
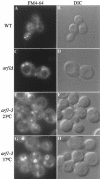
Immunofluorescent localization of Mnn1p to large ring structures in arf1Δ cells. Immunofluorescent staining of wild-type (SEY6210.5 pZV236, A), arf1Δ (6210.5 arf1Δ pZV236, C and G–I), and mnn1Δ (TGY122, E) cells using affinity-purified antibodies to Mnn1p was performed as described previously (Graham et al., 1994). (B, D, F, and J) Images captured using DIC optics and correspond to the adjacent fluorescent image. (G–I) Images captured at three different optical planes while focusing down through the cell (a Z-axis series). Arrows indicate ring structures visible in each optical plane. Rings were visible in 90% of the arf1Δ cells stained for Mnn1p.
Figure 5
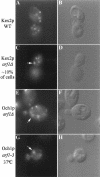
Immunofluorescent localization of Kex2p and Och1p in arf mutant cells. Wild-type (SEY6210.5 pKE2018) and arf1Δ (6210.5 arf1Δ pKE2018) cells overexpressing Kex2p and arf1Δ cells expressing Och1-HA (6210.5 arf1Δ pOH URA3) were stained using affinity-purified antibody to Kex2p (A and C) or a monoclonal antibody to the HA epitope tag (E). (B, D, and F) Images captured using DIC optics correspond to the adjacent fluorescent image. Ring structures were apparent in most arf1Δ cells stained for Och1-HA, whereas only ∼10% of arf1Δ cells exhibited rings when stained for Kex2p. (G and H) An arf1–3 ts mutant (strain C156–1B, G–H) transformed with pOH was preincubated for 1 h at 37°C before fixation and immunofluorescence localization of Och1p.
Figure 6
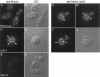
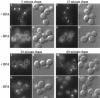
FM4–64 staining of endocytic membranes in wild-type and arf mutant cells. (A–D) Wild-type (SEY6210) and arf1Δ (6210 arf1Δ) cells were incubated in YPD containing 30 μM FM4–64 on ice for 45 min and then warmed to 25°C to initiate endocytosis as described previously (Vida and Emr, 1995). Cells were collected and applied to concanavalin A-treated slides 10 min after the temperature shift, and the images were acquired ∼10 min after cells were applied to the slides. (E–H) The arf1–3 ts mutant (C156–1B) was grown at 23°C and then half of the culture was shifted to 37°C and incubated for 1 h. Cells were harvested and resuspended at ∼2 × 108 cells/ml in YPD media prewarmed to 23°C or 37°C and incubated for 10 min. FM4–64 was added to 30 μM and incubated for 7 min, then the cells were harvested and resuspended in fresh prewarmed media and the incubation was continued. Images of the cells were acquired ∼15–20 min after addition of fresh media.
Figure 7
BFA perturbs transport of FM4–64 from endosomes to the vacuole. Strain TGY413–6D was preincubated at ∼2 × 108 cells/ml in YPD containing 50 mM Na HEPES (pH 7.0) for 15 min at 23°C. The culture was split in half and BFA was added to 100 μM final concentration to one culture (+BFA) and an equivalent aliquot of ethanol was added to the control culture (−BFA). The cells were incubated for 5 min before staining with FM4–64 for 7 min. The cells were then harvested and resuspended in fresh media (+/− BFA) to chase the FM4–64. Cells were applied to conconavalin A-treated slides at the chase times indicated and the images were acquired immediately.
Figure 8
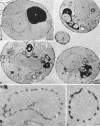
Electron microscopy of wild-type and arf1Δ cells. Cells were grown at 30°C and prepared for electron microscopy as described previously (Rieder et al., 1996). Representative thin sections through SEY6210 wild-type (A) and 6210_arf1Δ_ (B) cells are shown. (A and B) Positions of the nucleus (n), electron-dense vacuole (v), mitochondria (m), and lipid droplets (L) are indicated, and the bar represents 0.5 μm. In B (arf1Δ cells), arrows point to single-ring structures, arrowheads point to double or multiple ring structures (i.e., rings within rings), and asterisks are positioned next to reticulated membrane, which is often visualized as part of the rings. (C and D) Higher magnification photographs of representative ring structures observed in arf1Δ cells; the asterisk indicates reticulated membrane, and the bar represents 0.05 μm.
Comment in
- An MBoC favorite: ARF is required for maintenance of yeast Golgi and endosome structure and function.
Jackson CL. Jackson CL. Mol Biol Cell. 2012 Aug;23(15):2822. doi: 10.1091/mbc.E12-04-0255. Mol Biol Cell. 2012. PMID: 22848064 Free PMC article. No abstract available.
Similar articles
- COPI-independent anterograde transport: cargo-selective ER to Golgi protein transport in yeast COPI mutants.
Gaynor EC, Emr SD. Gaynor EC, et al. J Cell Biol. 1997 Feb 24;136(4):789-802. doi: 10.1083/jcb.136.4.789. J Cell Biol. 1997. PMID: 9049245 Free PMC article. - The ARF exchange factors Gea1p and Gea2p regulate Golgi structure and function in yeast.
Peyroche A, Courbeyrette R, Rambourg A, Jackson CL. Peyroche A, et al. J Cell Sci. 2001 Jun;114(Pt 12):2241-53. doi: 10.1242/jcs.114.12.2241. J Cell Sci. 2001. PMID: 11493664 - Retrograde transport from the yeast Golgi is mediated by two ARF GAP proteins with overlapping function.
Poon PP, Cassel D, Spang A, Rotman M, Pick E, Singer RA, Johnston GC. Poon PP, et al. EMBO J. 1999 Feb 1;18(3):555-64. doi: 10.1093/emboj/18.3.555. EMBO J. 1999. PMID: 9927415 Free PMC article. - Building a secretory apparatus: role of ARF1/COPI in Golgi biogenesis and maintenance.
Lippincott-Schwartz J, Cole NB, Donaldson JG. Lippincott-Schwartz J, et al. Histochem Cell Biol. 1998 May-Jun;109(5-6):449-62. doi: 10.1007/s004180050247. Histochem Cell Biol. 1998. PMID: 9681627 Review. - COPs regulating membrane traffic.
Kreis TE, Lowe M, Pepperkok R. Kreis TE, et al. Annu Rev Cell Dev Biol. 1995;11:677-706. doi: 10.1146/annurev.cb.11.110195.003333. Annu Rev Cell Dev Biol. 1995. PMID: 8689572 Review.
Cited by
- Dependence of phospholipase D1 multi-monoubiquitination on its enzymatic activity and palmitoylation.
Yin H, Gui Y, Du G, Frohman MA, Zheng XL. Yin H, et al. J Biol Chem. 2010 Apr 30;285(18):13580-8. doi: 10.1074/jbc.M109.046359. Epub 2010 Feb 26. J Biol Chem. 2010. PMID: 20189990 Free PMC article. - The Gcs1 and Age2 ArfGAP proteins provide overlapping essential function for transport from the yeast trans-Golgi network.
Poon PP, Nothwehr SF, Singer RA, Johnston GC. Poon PP, et al. J Cell Biol. 2001 Dec 24;155(7):1239-50. doi: 10.1083/jcb.200108075. Epub 2001 Dec 17. J Cell Biol. 2001. PMID: 11756474 Free PMC article. - Multiple roles of Arf1 GTPase in the yeast exocytic and endocytic pathways.
Yahara N, Ueda T, Sato K, Nakano A. Yahara N, et al. Mol Biol Cell. 2001 Jan;12(1):221-38. doi: 10.1091/mbc.12.1.221. Mol Biol Cell. 2001. PMID: 11160834 Free PMC article. - A novel combinatorial approach of quantitative microscopy and in silico modeling deciphers Arf1-dependent Golgi size regulation.
Iyer P, Sutradhar S, Paul R, Bhattacharyya D. Iyer P, et al. Eur Phys J E Soft Matter. 2019 Dec 12;42(12):154. doi: 10.1140/epje/i2019-11920-x. Eur Phys J E Soft Matter. 2019. PMID: 31834534 - Requirement for neo1p in retrograde transport from the Golgi complex to the endoplasmic reticulum.
Hua Z, Graham TR. Hua Z, et al. Mol Biol Cell. 2003 Dec;14(12):4971-83. doi: 10.1091/mbc.e03-07-0463. Epub 2003 Sep 5. Mol Biol Cell. 2003. PMID: 12960419 Free PMC article.
References
- Bankaitis V, Aitken J, Cleves A, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. - PubMed
- Boman AL, Kahn RA. Arf proteins: the membrane traffic police? Trends Biochem Sci. 1995;20:147–150. - PubMed
- Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. - PubMed
- Cockcroft S, Thomas GM, Fensome A, Geny B, Cunningham E, Gout I, Hiles I, Totty NF, Truong O, Hsuan JJ. Phospholipase D: a downstream effector of ARF in granulocytes. Science. 1994;263:523–526. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials