The physical association of multiple molecular chaperone proteins with mutant p53 is altered by geldanamycin, an hsp90-binding agent - PubMed (original) (raw)
The physical association of multiple molecular chaperone proteins with mutant p53 is altered by geldanamycin, an hsp90-binding agent
L Whitesell et al. Mol Cell Biol. 1998 Mar.
Abstract
Wild-type p53 is a short-lived protein which turns over very rapidly via selective proteolysis in the ubiquitin-proteasome pathway. Most p53 mutations, however, encode for protein products which display markedly increased intracellular levels and are associated with positive tumor-promoting activity. The mechanism by which mutation leads to impairment of ubiquitination and proteasome-mediated degradation is unknown, but it has been noted that many transforming p53 mutants are found in stable physical association with molecular chaperones of the hsp70 class. To explore a possible role for aberrant chaperone interactions in mediating the altered function of mutant p53 and its intracellular accumulation, we examined the chaperone proteins which physically associate with a temperature-sensitive murine p53 mutant. In lysate prepared from A1-5 cells grown under mutant temperature conditions, hsp70 coprecipitated with p53Val135 as previously reported by others, but in addition, other well-recognized elements of the cellular chaperone machinery, including hsp90, cyclophilin 40, and p23, were detected. Under temperature conditions favoring wild-type p53 conformation, the coprecipitation of chaperone proteins with p53 was lost in conjunction with the restoration of its transcriptional activating activity. Chaperone interactions similar to those demonstrated in A1-5 cells under mutant conditions were also detected in human breast cancer cells expressing two different hot-spot mutations. To examine the effect of directly disrupting chaperone interactions with mutant p53, we made use of geldanamycin (GA), a selective hsp90-binding agent which has been shown to alter the chaperone associations regulating the function of unliganded steroid receptors. GA treatment of cells altered heteroprotein complex formation with several different mutant p53 species. It increased p53 turnover and resulted in nuclear translocation of the protein in A1-5 cells. GA did not, however, appear to restore wild-type transcriptional activating activity to mutant p53 proteins in either A1-5 cells or human breast cancer cell lines.
Figures
FIG. 1
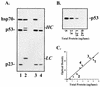
Mutant p53 is physically associated with multiple molecular chaperones. A1-5 cells were grown at 39°C (mutant conditions), and lysates were prepared in buffer containing sodium molybdate (10 mM) and/or monothioglycerol (10 mM) as indicated. Anti-p53 IP was performed with PAb421, and precipitates were analyzed by immunoblotting for the presence of the indicated chaperone proteins. Total lysate (lane T) was analyzed to verify the presence and migration position of each chaperone. LC, antibody light chain.
FIG. 2
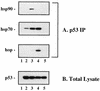
Most of the mutant p53 in A1-5 cells is complexed with molecular chaperones. Cells were grown at 39°C, and lysates were prepared in molybdate-containing buffer. (A) IPs were performed on replicate aliquots by using control mouse IgG (lanes 1 and 3), anti-p23 antibody (JJ3; lane 2), or anti-hsp70 antibody (BB70; lane 4) in order to deplete the lysate of the relevant chaperone. Aliquots of immunodepleted supernatant were then analyzed for remaining hsp70, p53, and p23 content by Western blotting. HC and LC indicate the positions of residual antibody heavy chain and light chain, respectively, remaining in some of the supernatants after IP. (B) Aliquots of cell lysate containing the indicated amounts of total protein were immunoblotted on the same membrane as the samples depicted in panel A in order to generate a standard curve for the estimation of p53 levels. The position of p53 as detected by PAb421 is indicated. (C) Scanning densitometry (Bio-Rad GS-700 instrument and Molecular Analyst software) was performed to quantitate the p53 signals displayed in panels A and B. The optical densities (arbitrary units) of the bands depicted in panel B are plotted on the y axis, while the corresponding amounts of total cellular protein are plotted on the x axis. A linear curve fit (_r_2 = 0.98) for the data points is shown as a solid line. Numbered arrows indicate the optical densities of the p53 bands visible in each of the corresponding lanes displayed in panel A.
FIG. 3
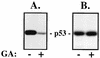
GA treatment of cells alters the composition of chaperone complexes coprecipitating with p53 in a manner distinct from temperature shift. A1-5 cells were incubated at 32°C (lanes 2) or 39°C (lanes 3 to 5) for 6 h, followed by the addition of 1.8 μM GA (lanes 4) or an equal volume of dimethyl sulfoxide vehicle (lanes 2, 3, and 5) for 10 min prior to lysis in molybdate-containing buffer. As a control, PC-3M carcinoma cells, which do not express p53, were lysed in the same buffer (lane 1). (A) IP with PAb421 (lanes 1 to 4) or irrelevant isotype-matched mouse IgG (lanes 5) was performed, and precipitates were analyzed by immunoblotting for the presence of the indicated chaperones. (B) Total proteins (25 μg) from the lysates analyzed in panel A were analyzed by immunoblotting for the level of p53 present.
FIG. 4
IP of p53 with chaperone complexes is not epitope dependent. (A) A1-5 cells were incubated at 32°C (lanes 1 and 2) or 39°C (lanes 3 and 4) for 6 h, followed by the addition of 1.8 μM GA (lanes 2 and 4) or control vehicle (lanes 1 and 3) for 10 min prior to lysis as described in the legend to Fig. 3. Anti-p23 IP with monoclonal antibody JJ3 was performed, and precipitates were analyzed by immunoblotting for the presence of the indicated proteins. HC refers to the position of the immunoglobulin heavy chain, which was used for IP and detected by the secondary antibody used in the immunoblotting procedure. (B) A1-5 cells were incubated at 39°C for 6 h followed by the addition of GA (lanes 4 and 5) or control vehicle (lanes 1 to 3) for 10 min and lysis as described for panel A. p53 IP was performed with PAb242 (lanes 1 and 4), PAb421 (lanes 2 and 5), or no primary antibody (lane 3). Precipitates were analyzed by immunoblotting for the presence of hsp70 and hsp90 as indicated.
FIG. 5
Certain p53 mutants are stably associated with molecular chaperones in human breast cancer cells. Subconfluent cultures of the cell lines MCF-7 (wild-type p53), T47D (mutant p53, codon 194), MDA-MB-468 (mutant, codon 273), and SkBr3 (mutant, codon 175) were lysed in molybdate-containing buffer with or without prior incubation at 37°C in medium containing GA (1.8 μM) for 15 min as indicated. Anti-p53 IP was performed with antibody DO-1. Control IP consisted of mouse IgG at the same concentration and lysate from non-GA-treated T47D cells. Precipitates were analyzed by immunoblotting for the presence of the indicated chaperones. LC refers to the position of the antibody light chain used for IP and detected by the secondary antibody used in the blotting procedure.
FIG. 6
GA treatment of A1-5 cells decreases p53 levels without altering its rate of synthesis. Cells were cultured overnight at 39°C in the presence or absence of GA (1.8 μM) as indicated. (A) Cells were lysed, and the level of p53 in equal amounts of total protein was evaluated by immunoblotting. (B) Cells were metabolically labeled with [35S]methionine for 1 h, followed by lysis and IP of p53 with PAb421 from equal amounts of trichloroacetic acid-precipitable material. Precipitates were fractionated by SDS-PAGE and visualized by autoradiography.
FIG. 7
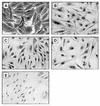
GA treatment of A1-5 cells induces nuclear localization of mutant p53. Cells growing on coverslips were incubated at 39°C (A, C, and E) or 32°C (B and D) for 6 h in the presence (C and D) or absence (A, B, and E) of GA. Following fixation in cold methanol-acetone, p53 localization was visualized by indirect immunocytochemistry using PAb421 except for panel E, where an irrelevant control antibody was applied. The dark diaminobenzidine signal represents p53 immunoreactivity. All panels were photographed at the same magnification and exposure settings.
FIG. 8
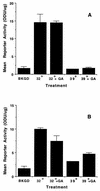
GA treatment of A1-5 cells does not restore wild-type p53 transcriptional activating activity. Cells were stably transfected with the p53-responsive reporter construct PG13/βgal (A) or WAF1/βgal (B). The level of β-galactosidase activity was quantitated in cell lysates following overnight incubation with or without GA at the indicated temperatures. BKGD refers to the level of β-galactosidase activity measured in nontransfected A1-5 cells. All determinations were performed in triplicate, and mean values are depicted, with the standard deviations of the means indicated by error bars. The results presented are derived from analysis of a clonal isolate derived from transfections with each of the two vectors, but similar results were obtained in assays using pooled colonies. ODU, optical density units.
FIG. 9
GA treatment does not induce mdm-2 expression in A1-5 cells. Cells were incubated overnight at the indicated temperatures with or without the addition of GA (1.8 μM). Lysates were prepared, and the induction of mdm-2 was detected by immunoblotting with monoclonal antibody 2A10.
FIG. 10
GA treatment does not induce WAF-1 expression in human breast cancer cells. Subconfluent cultures of the indicated cell lines were treated overnight with control vehicle, doxorubicin (Dox; 0.2 or 1.0 μM), or GA (1.8 μM). Lysates were fractionated by SDS-PAGE, and proteins were transferred to nitrocellulose. The upper half of the membrane was probed with anti-p53 antibody DO-1, while the lower half was probed with antiserum to human WAF-1.
References
- Abarzua P, LoSardo J E, Gubler M L, Neri A. Microinjection of monoclonal antibody PAb421 into human SW480 colorectal carcinoma cells restores the transcriptional activation function to mutant p53. Cancer Res. 1995;55:3490–3494. - PubMed
- Blagosklonny M V, Toretsky J, Neckers L. Geldanamycin selectively destabilizes and conformationally alters mutated p53. Oncogene. 1995;11:933–939. - PubMed
- Chen S, Prapapanich V, Rimerman R A, Honore B, Smith D F. Interactions of p60, a mediator of progesterone receptor assembly, with heat shock proteins hsp90 and hsp70. Mol Endocrinol. 1996;10:682–693. - PubMed
- Chernov M V, Stark G. The p53 activation and apoptosis induced by DNA damage are reversibly inhibited by salicylate. Oncogene. 1997;14:2503–2510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous