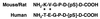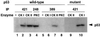Functional activation of p53 via phosphorylation following DNA damage by UV but not gamma radiation - PubMed (original) (raw)
Comparative Study
Functional activation of p53 via phosphorylation following DNA damage by UV but not gamma radiation
M Kapoor et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The tumor suppressor p53 is a nuclear phosphoprotein in which DNA-binding activity is increased on exposure to DNA-damaging agents such as UV or gamma radiation by unknown mechanisms. Because phosphorylation of p53 at the casein kinase (CK) II site activates p53 for DNA-binding function in vitro, we sought to determine the in vivo relevance of phosphorylation at this site after UV and gamma radiation. A polyclonal antibody was generated that binds to bacterially expressed p53 only when phosphorylated in vitro by CK II. Using this antibody, we showed that p53 is phosphorylated at the CK II site upon UV treatment of early passage rat embryo fibroblasts and RKO cells. In addition, DNA-binding assays indicated that phosphorylated p53 bound to a p53-responsive element, suggesting functional activation. However, gamma radiation, which also stabilizes p53, did not result in phosphorylation at the CK II site. These results indicate that phosphorylation at the CK II site is one of the post-translational mechanisms through which p53 is activated in response to UV radiation and that different mechanisms activate p53 after DNA damage by gamma radiation.
Figures
Figure 1
Sequence comparison of the C-terminal phosphopeptide of murine p53 used for generating Ab389 (QCB) with the C-terminal sequence of human p53.
Figure 2
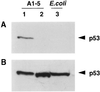
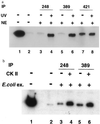
Ab389 does not bind to dephosphorylated murine p53. (A) Extracts from A1–5 cells expressing a wild-type p53 at the permissive temperature of 32°C were treated with CIP at 37°C for 1 h. Untreated (lane 1) and CIP-treated extracts (lane 2) were then resolved on 10% SDS/PAGE followed by Western blotting using Ab389. Enhanced chemiluminescence kit (Amersham) was used to detect antibody-specific proteins. Extracts from E. coli cells expressing human p53 were run in lane 3. (B) The Western blot from a was stripped and reprobed with p53-specific mAb, pAb421.
Figure 3
Ab389 binds human p53 when phosphorylated in vitro by CK II. Extracts from E. coli cells expressing wild-type (lanes 1–8) or mutant (serine to alanine substitution at 392; lanes 9 and 10) human p53 were used for in vitro phosphorylation reactions. Extracts were treated with CK I (lanes 2, 6, and 10), CK II (lanes 3, 7, and 9), or with PKC (lanes 4 and 8). All reactions were supplemented with 200 μCi/mmol of [γ-32P]ATP and were performed at 30°C for 30 min followed by immunoprecipitation by using pAb421 (lanes 1–3 and 9–10), pAb248 (lane 4), or Ab389 (lanes 5–8) and then SDS/PAGE analysis. Lanes 1 and 5 are controls in which no enzyme was added in the phosphorylation reactions.
Figure 4
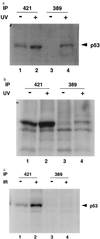
Ab389 binds to p53 from UV-treated cells. RKO cells (a) and early passage rat embryo fibroblasts (b) were treated with 10 J/m2 (30 J/m2 for RKO cells) and metabolically labeled with [S35]methionine and [S35]cysteine for 30 min, 24 h (2 h for RKO cells) after radiation. Cells were harvested and lysed as described (14). Extracts from untreated (lanes 1 and 3) and UV-treated (lanes 2 and 4) cells were used for immunoprecipitation with pAb421 (lanes 1 and 2) or Ab389 (lanes 3 and 4) followed by SDS/PAGE and autoradiography. (c) RKO cells were treated with 6 Gy of γ radiation. Four hours after radiation, cells were metabolically labeled and harvested as described above. Extracts from these cells were used for immunoprecipitation with pAb421 (lanes 1 and 2) or Ab389 (lanes 3 and 4) followed by SDS/PAGE and autoradiography.
Figure 5
p53 bound to DNA is immunoprecipitated by Ab389. (a) Nuclear extracts (NE) were prepared from UV-treated and untreated rat embryo fibroblasts, and DNA-binding assays were performed as described (20). Extracts from untreated (lanes 3, 5, and 7) and UV-treated cells (lanes 4, 6, and 8) were incubated with the radiolabeled DNA containing the p21 promoter (20) for 30 min at 4°C. DNA-protein complexes were immunoprecipitated by either pAb248 (lanes 3 and 4), Ab389 (lanes 5 and 6), or pAb421 (lanes 7 and 8), and the bound DNA was analyzed on 5% nondenaturing polyacrylamide gel followed by autoradiography. Lanes 1 and 2 contain free probe and no nuclear extract, respectively. (b) Bacterially expressed p53 was phosphorylated in vitro with CK II (as described in Fig. 3). Untreated and phosphorylated extracts were used for DNA-binding assays as described above. Immunoprecipitations were performed with pAb248 (lanes 3 and 4) or Ab389 (lanes 5 and 6), and bound DNA was analyzed as above. Lanes 1 and 2 contain free probe and no nuclear extract, respectively.
Similar articles
- Cooperative phosphorylation at multiple sites is required to activate p53 in response to UV radiation.
Kapoor M, Hamm R, Yan W, Taya Y, Lozano G. Kapoor M, et al. Oncogene. 2000 Jan 20;19(3):358-64. doi: 10.1038/sj.onc.1203300. Oncogene. 2000. PMID: 10656682 - DNA damage triggers DRB-resistant phosphorylation of human p53 at the CK2 site.
Blaydes JP, Hupp TR. Blaydes JP, et al. Oncogene. 1998 Aug 27;17(8):1045-52. doi: 10.1038/sj.onc.1202014. Oncogene. 1998. PMID: 9747884 - Ultraviolet radiation, but not gamma radiation or etoposide-induced DNA damage, results in the phosphorylation of the murine p53 protein at serine-389.
Lu H, Taya Y, Ikeda M, Levine AJ. Lu H, et al. Proc Natl Acad Sci U S A. 1998 May 26;95(11):6399-402. doi: 10.1073/pnas.95.11.6399. Proc Natl Acad Sci U S A. 1998. PMID: 9600977 Free PMC article. - Regulation of p53 in response to DNA damage.
Lakin ND, Jackson SP. Lakin ND, et al. Oncogene. 1999 Dec 13;18(53):7644-55. doi: 10.1038/sj.onc.1203015. Oncogene. 1999. PMID: 10618704 Review. - New developments in the multi-site phosphorylation and integration of stress signalling at p53.
Meek DW. Meek DW. Int J Radiat Biol. 1998 Dec;74(6):729-37. doi: 10.1080/095530098141005. Int J Radiat Biol. 1998. PMID: 9881718 Review.
Cited by
- Ets1 is required for p53 transcriptional activity in UV-induced apoptosis in embryonic stem cells.
Xu D, Wilson TJ, Chan D, De Luca E, Zhou J, Hertzog PJ, Kola I. Xu D, et al. EMBO J. 2002 Aug 1;21(15):4081-93. doi: 10.1093/emboj/cdf413. EMBO J. 2002. PMID: 12145208 Free PMC article. - Application of protein lysate microarrays to molecular marker verification and quantification.
Ramaswamy A, Lin E, Chen I, Mitra R, Morrisett J, Coombes K, Ju Z, Kapoor M. Ramaswamy A, et al. Proteome Sci. 2005 Nov 10;3:9. doi: 10.1186/1477-5956-3-9. Proteome Sci. 2005. PMID: 16281978 Free PMC article. - Activation and activities of the p53 tumour suppressor protein.
Bálint E E, Vousden KH. Bálint E E, et al. Br J Cancer. 2001 Dec 14;85(12):1813-23. doi: 10.1054/bjoc.2001.2128. Br J Cancer. 2001. PMID: 11747320 Free PMC article. Review. - p53 accumulates but is functionally impaired when DNA synthesis is blocked.
Gottifredi V, Shieh S, Taya Y, Prives C. Gottifredi V, et al. Proc Natl Acad Sci U S A. 2001 Jan 30;98(3):1036-41. doi: 10.1073/pnas.98.3.1036. Epub 2001 Jan 23. Proc Natl Acad Sci U S A. 2001. PMID: 11158590 Free PMC article. - ARF differentially modulates apoptosis induced by E2F1 and Myc.
Russell JL, Powers JT, Rounbehler RJ, Rogers PM, Conti CJ, Johnson DG. Russell JL, et al. Mol Cell Biol. 2002 Mar;22(5):1360-8. doi: 10.1128/MCB.22.5.1360-1368.2002. Mol Cell Biol. 2002. PMID: 11839803 Free PMC article.
References
- Finlay C A, Hinds P W, Levine A J. Cell. 1989;57:1083–1093. - PubMed
- Chen P-L, Chen Y M, Bookstein R, Lee W-H. Science. 1990;250:1576–1580. - PubMed
- Baker S J, Markowitz S, Fearon E R, Willson J K, Vogelstein B. Science. 1990;249:912–915. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous