Monocyte chemoattractant protein-1-induced CCR2B receptor desensitization mediated by the G protein-coupled receptor kinase 2 - PubMed (original) (raw)
Monocyte chemoattractant protein-1-induced CCR2B receptor desensitization mediated by the G protein-coupled receptor kinase 2
A M Aragay et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Monocyte chemoattractant protein 1 (MCP-1) is a member of the chemokine cytokine family, whose physiological function is mediated by binding to the CCR2 and CCR4 receptors, which are members of the G protein-coupled receptor family. MCP-1 plays a critical role in both activation and migration of leukocytes. Rapid chemokine receptor desensitization is very likely essential for accurate chemotaxis. In this report, we show that MCP-1 binding to the CCR2 receptor in Mono Mac 1 cells promotes the rapid desensitization of MCP-1-induced calcium flux responses. This desensitization correlates with the Ser/Thr phosphorylation of the receptor and with the transient translocation of the G protein-coupled receptor kinase 2 (GRK2, also called beta-adrenergic kinase 1 or betaARK1) to the membrane. We also demonstrate that GRK2 and the uncoupling protein beta-arrestin associate with the receptor, forming a macromolecular complex shortly after MCP-1 binding. Calcium flux responses to MCP-1 in HEK293 cells expressing the CCR2B receptor were also markedly reduced upon cotransfection with GRK2 or the homologous kinase GRK3. Nevertheless, expression of the GRK2 dominant-negative mutant betaARK-K220R did not affect the initial calcium response, but favored receptor response to a subsequent challenge by agonists. The modulation of the CCR2B receptor by GRK2 suggests an important role for this kinase in the regulation of monocyte and lymphocyte response to chemokines.
Figures
Figure 1
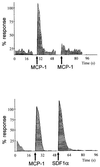
MCP-1-induced desensitization of the CCR2B receptor. Calcium influx was promoted by the addition of 10 nM human MCP-1 to Mono Mac 1 cells loaded with Fluo-3; a second challenge with 10 nM MCP-1 promoted very little calcium response (Upper). As a control, MCP-1-induced cells were stimulated with 10 nM SDF-1α (Lower). Calcium influx in response to MCP-1 was determined by flow cytometry at 525 nm, as detailed in the text. Data are given as a percentage of maximum MCP-1 fluorescence response. Arrows depict the time of chemokine addition.
Figure 2
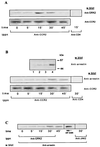
(A) Time course of serine/threonine phosphorylation of the CCR2B receptor in Mono Mac 1 cells. Mono Mac 1 cells were stimulated for the times indicated and lysed, and cell extracts were immunoprecipitated with an anti-CCR2 antibody. After SDS/PAGE and transfer, the same blot was first developed with a mixture of anti-phosphoserine/threonine antibodies (Upper) and then reprobed with the anti-CCR2 antibody MCP-1R05 (Lower). The molecular mass of the CCR2 receptor is 38 kDa. The figure is representative of three experiments with similar results. (B) Western blot analysis of the presence of GRK2 and GRK3 in Mono Mac 1 cell extracts (lane 1). For comparative purposes, the same analysis was performed in HEK293 cells transiently transfected with pcDNA3, GRK2, GRK3, GRK5, or GRK6 (lanes 2–6, respectively). Blots were tested with the anti-GRK2 AB9 (Left) and anti-GRK3 antibodies (Right). The molecular mass of GRK2 and GRK3 is ≈80 kDa and 78 kDa, respectively. (C) Western blot analysis of MCP-1-induced GRK2 translocation in Mono Mac 1 cells. Cells were stimulated with MCP-1 for the times indicated and particulate fractions were obtained as described in the text. Equal protein amounts were resolved in SDS/PAGE, blotted, and developed with anti-GRK2 AB9 antibody (Upper). Data were quantitated by laser densitometry, normalized by the signal of an unrelated band, and represented as the percentage of particulate GRK2 before stimulation (Lower). This experiment was repeated twice with similar results.
Figure 3
Association of GRK2 and β-arrestin to the MCP-1-stimulated CCR2B receptor in Mono Mac 1 cells. (A and B) Whole cell lysates from Mono Mac 1 cells stimulated with MCP-1 for the times indicated were precipitated with anti-CCR2 MCP-1R03 antibody. Immunoprecipitates were first analyzed in a Western blot with anti-GRK2 AB9 (A Top) or with anti-arrestin (B Middle) antibodies, and the same blots were then developed with anti-CCR2 MCP-1R05 (A and B Lower) antibodies, as indicated. In B Upper, lysates from mock or β-arrestin1-transfected HEK293 cells (lanes 1 and 2, respectively) or Mono Mac 1 particulate and cytosolic fractions (lanes 3 and 4, respectively), were analyzed in a Western blot with an anti-arrestin mAb. The molecular mass of the β-arrestin1 is ≈50 kDa. (C) MCP-1-stimulated Mono Mac 1 cell lysates were immunoprecipitated with anti-GRK2 antibody and analyzed by Western blotting with anti-arrestin antibody. A Mono Mac 1 cell lysate (MM-1) was included as a control of β-arrestin migration in the same gel. As negative controls, MCP-1-stimulated cell lysates were immunoprecipitated with an anti-CD4 mAb (A and B) or anti-JAK2 polyclonal antibodies (C), and Western blots were stained with the indicated antibody. Results are representative of three experiments with similar results.
Figure 4
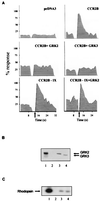
GRK2 and GRK3 modulation of MCP-1 signaling mediated by the CCR2B receptor. (A) HEK293 cells were transiently transfected with pcDNA3 alone; with pcDNACCR2B with pcDNA3, pcDNAGRK2, or pCMVGRK3; or with pcDNACCR2B-IX and pcDNA3 or pcDNAGRK2 as indicated. Calcium was determined as described in the legend of Fig. 1. Data are given as a percentage of maximal MCP-1-induced response. Arrows indicate the time of MCP-1 addition. (B) Western blot analysis of the expression level of GRK2 and GRK3 in transiently cotransfected HEK293 cells. Recombinant GRK2 (lane 1) and lysates from CCR2B, CCR2B + GRK2, and CCR2B + GRK3 cells (lanes 2–4, respectively) were resolved by SDS/PAGE and transferred, and blots were developed simultaneously with anti-GRK2 AB9 and anti-GRK3 antibodies. (C) HEK293 cells cotransfected with the CCR2B receptor alone (lane 2) or with either GRK2 (lane 3) or GRK3 (lane 4) were lysed, and the cytosolic kinase activity was determined with rhodopsin as substrate. Recombinant GRK2 (10 nM) was used as a control (lane 1). A representative autoradiogram is shown. Results in all panels are representative of two independent experiments.
Figure 5
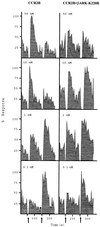
βARK-K220R inhibition of MCP-1-induced desensitization of the CCR2B receptor. HEK293 cells were transiently cotransfected with pcDNACCR2B and either empty vector or pcDNAβARK-K220R. Cells were stimulated first with different concentrations of MCP-1 as indicated. After approximately 2 min, 10 nM MCP-1 was added a second time as indicated by the arrows. Calcium was determined as described in the legend of Fig. 1. Data are given as a percentage of maximal MCP-1-induced and are representative of two independent experiments.
Similar articles
- The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor.
Mellado M, Rodríguez-Frade JM, Aragay A, del Real G, Martín AM, Vila-Coro AJ, Serrano A, Mayor F Jr, Martínez-A C. Mellado M, et al. J Immunol. 1998 Jul 15;161(2):805-13. J Immunol. 1998. PMID: 9670957 - Effect of different G protein-coupled receptor kinases on phosphorylation and desensitization of the alpha1B-adrenergic receptor.
Diviani D, Lattion AL, Larbi N, Kunapuli P, Pronin A, Benovic JL, Cotecchia S. Diviani D, et al. J Biol Chem. 1996 Mar 1;271(9):5049-58. doi: 10.1074/jbc.271.9.5049. J Biol Chem. 1996. PMID: 8617782 - G protein-coupled receptor kinase 2 (GRK2): mechanisms of regulation and physiological functions.
Aragay AM, Ruiz-Gómez A, Penela P, Sarnago S, Elorza A, Jiménez-Sainz MC, Mayor F Jr. Aragay AM, et al. FEBS Lett. 1998 Jun 23;430(1-2):37-40. doi: 10.1016/s0014-5793(98)00495-5. FEBS Lett. 1998. PMID: 9678590 Review. - GTP-binding-protein-coupled receptor kinases--two mechanistic models.
Palczewski K. Palczewski K. Eur J Biochem. 1997 Sep 1;248(2):261-9. doi: 10.1111/j.1432-1033.1997.00261.x. Eur J Biochem. 1997. PMID: 9346277 Review.
Cited by
- Hematopoietic G-protein-coupled receptor kinase 2 deficiency decreases atherosclerotic lesion formation in LDL receptor-knockout mice.
Otten JJ, de Jager SC, Kavelaars A, Seijkens T, Bot I, Wijnands E, Beckers L, Westra MM, Bot M, Busch M, Bermudez B, van Berkel TJ, Heijnen CJ, Biessen EA. Otten JJ, et al. FASEB J. 2013 Jan;27(1):265-76. doi: 10.1096/fj.12-205351. Epub 2012 Oct 9. FASEB J. 2013. PMID: 23047899 Free PMC article. - Filamin a binds to CCR2B and regulates its internalization.
Minsaas L, Planagumà J, Madziva M, Krakstad BF, Masià-Balagué M, Katz AA, Aragay AM. Minsaas L, et al. PLoS One. 2010 Aug 17;5(8):e12212. doi: 10.1371/journal.pone.0012212. PLoS One. 2010. PMID: 20808917 Free PMC article. - G Protein-Coupled Receptor Kinases in the Inflammatory Response and Signaling.
Steury MD, McCabe LR, Parameswaran N. Steury MD, et al. Adv Immunol. 2017;136:227-277. doi: 10.1016/bs.ai.2017.05.003. Epub 2017 Jun 10. Adv Immunol. 2017. PMID: 28950947 Free PMC article. Review. - An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorigenesis.
Jin G, Kawsar HI, Hirsch SA, Zeng C, Jia X, Feng Z, Ghosh SK, Zheng QY, Zhou A, McIntyre TM, Weinberg A. Jin G, et al. PLoS One. 2010 Jun 8;5(6):e10993. doi: 10.1371/journal.pone.0010993. PLoS One. 2010. PMID: 20544025 Free PMC article. - Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues.
Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. Palframan RT, et al. J Exp Med. 2001 Nov 5;194(9):1361-73. doi: 10.1084/jem.194.9.1361. J Exp Med. 2001. PMID: 11696600 Free PMC article.
References
- Murphy P M. Annu Rev Immunol. 1994;12:593–633. - PubMed
- Schall T J. In: The Cytokine Handbook. Thompson A W, editor. London: Academic; 1994. pp. 419–460.
- Howard O M Z, Ben-Baruch A, Oppenheim J J. Trends Biotechnol. 1996;14:46–51. - PubMed
- Hedrick J A, Zlotnik A. Curr Opin Immunol. 1996;8:343–347. - PubMed
- Peri G, Milanese C, Matteucci C, Ruco L, Zhou D, Sossanis S, Coletta I, Mantovani A. J Immunol Methods. 1994;174:249–257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous