The T cell leukemia LIM protein Lmo2 is necessary for adult mouse hematopoiesis - PubMed (original) (raw)
The T cell leukemia LIM protein Lmo2 is necessary for adult mouse hematopoiesis
Y Yamada et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The LIM-finger protein Lmo2, which is activated in T cell leukemias by chromosomal translocations, is required for yolk sac erythropoiesis. Because Lmo2 null mutant mice die at embryonic day 9-10, it prevents an assessment of a role in other stages of hematopoiesis. We have now studied the hematopoietic contribution of homozygous mutant Lmo2 -/- mouse embryonic stem cells and found that Lmo2 -/- cells do not contribute to any hematopoietic lineage in adult chimeric mice, but reintroduction of an Lmo2-expression vector rescues the ability of Lmo2 null embryonic stem cells to contribute to all lineages tested. This disruption of hematopoiesis probably occurs because interaction of Lmo2 protein with factors such as Tal1/Scl is precluded. Thus, Lmo2 is necessary for early stages of hematopoiesis, and the Lmo2 master gene encodes a protein that has a central and crucial role in the hematopoietic development.
Figures
Figure 1
Requirement for Lmo2 expression in hematopoiesis in vitro. ES cells were differentiated in the presence of interleukin 1α, stem cell factor, interleukin 3, and erythropoietin, and PCR amplifications were performed with cDNA synthesized from RNA isolated from embryoid bodies at day 0 and at 4 and 10 days after induction, with gene-specific primers for the indicated transcripts. Actin was used as a quality control for the RNA prepared from ES cells. ES cells examined were wild-type (+/+), Lmo2 −/−, and Lmo2 −/− into which an Lmo2 expression vector had been transfected (Lmo2 −/−R). Sizes of the transcripts are indicated.
Figure 2
Lmo2 −/− ES cells cannot differentiate into hematopoietic tissues in vivo. (A) Tissue contribution of Lmo2 +/− and −/− ES cells in chimeric mice. Tissue extracts from chimeric mice were prepared and the relative proportion of GPI isozymes 1B (C57BL/6 blastocysts) or 1A (129 ES cells) was assayed. Chimeric mice were generated by injecting Lmo2 +/− ES cells (C320) or two Lmo2 −/− ES cell clones, 71(−/−) and 53(−/−). (B) PCR amplifications were performed with the polymorphic microsatellite marker D10Mit180 (15) and DNA isolated from tissues of chimeric mice. PCR products differ between C57BL/6 (blastocysts) and 129 (ES cells), as seen in products from a 50:50 mixture of C57BL/6 and 129 DNA.
Figure 3
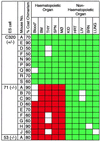
Contribution of the Lmo2 +/− (C320) and −/− (clones 71 and 53) ES cells to tissues in chimeric mice. Individual mice are indicated and the approximate coat color chimerism is given. The mouse tissue was assayed with combinations of techniques. In each case, GPI analysis was first performed and these data were confirmed by the microsatellite PCR method (complete concordance was obtained). For C320 thymus sample E, no GPI data is available, the results being obtained from microsatellite and Y chromosome PCR only. Blood (∗) analysis was carried out by the modified globin analysis (13) and was only performed on C320 chimeras A, E, D, F, N, P, R, and S; clone 71 chimeras D, E, F, G, H, and J; and clone 53 chimera A. Boxes: green, ES contribution was detectable; red, no ES contribution was detectable; white, no information. Data from those with more than 50% coat color chimerism are shown. THY, thymic cells; SPN, splenic cells; MØ, peritoneal macrophage; KID, kidney; HRT, heart; LIV, liver; BRN, brain.
Figure 4
Hematopoietic rescue by reexpression of Lmo2 −/− ES cells. (A) PCR amplifications were performed with Y chromosome-specific primers (giving the Uty product) and DNA isolated from 24-h cultures of peritoneal macrophages from female chimeric mice produced with the Lmo2 −/− ES clone 71 (mice 71H and 71J) or the BR12 _Lmo2_-expressing −/− clone (−/−R; mouse 12H). The PCR products were separated on 4% Nusieve agarose. DNA quality for each PCR was tested simultaneously with the microsatellite primer pair D10Mit180 (data not shown). Electrophoresis of control PCRs using DNA samples from a male mouse and from ES cells are also shown. (B) Modified hemoglobin analysis of blood samples (13) of Lmo2 −/− clone 71 (71E and 71F) and clone BR12 (12F, 12G, and 12H) chimeric mice. Hemoglobins were separated on cellulose acetate thin-layer plates. Hbbs is specific for C57BL/6 (blastocysts) and Hbbd is specific for 129 (ES cells) as indicated by arrows. Note the relatively faint ES cell-specific bands seen in BR12 samples; this phenomenon was also observed in the Tal1/Scl rescue experiments (20) and is possibly due to the lack of proper regulation of gene expression (e.g., perhaps a promoter efficiency effect or a chromosome location effect on Lmo2 expression).
Figure 5
Rescues in all components of hematopoiesis by Lmo2 reconstitution. (A) Similar PCR analyses were performed, as in Fig. 4, with DNA from purified splenic cells and purified thymic cells, and the products were fractionated on 4% Nusieve agarose gels. Three chimeras were analyzed from the Lmo2 −/− ES clone 71 (71A, 71H, and 71J) and one chimera made from ES clone BR12 (−/−R; 12C). (B) Summary of ES cell contribution of the Lmo2 −/− clones in which Lmo2 expression has been restored by using an Lmo2 expression vector (BR3 and BR12). Individual chimeric mice were analyzed, as shown, and the sex and estimated level of coat color chimerism are listed. Green boxes indicate ES contribution was detectable and the method applied is shown (M, microsatellite PCR; g, globin; G, GPI; Y, Y chromosome PCR). White boxes indicate no ES contribution was detectable. THY, thymic cells; SPN, splenic cells; MØ, peritoneal macrophage; KID, kidney; HRT, heart; LIV, liver; BRN, brain.
Similar articles
- The oncogenic LIM-only transcription factor Lmo2 regulates angiogenesis but not vasculogenesis in mice.
Yamada Y, Pannell R, Forster A, Rabbitts TH. Yamada Y, et al. Proc Natl Acad Sci U S A. 2000 Jan 4;97(1):320-4. doi: 10.1073/pnas.97.1.320. Proc Natl Acad Sci U S A. 2000. PMID: 10618416 Free PMC article. - A DNA-binding mutant of TAL1 cooperates with LMO2 to cause T cell leukemia in mice.
Draheim KM, Hermance N, Yang Y, Arous E, Calvo J, Kelliher MA. Draheim KM, et al. Oncogene. 2011 Mar 10;30(10):1252-60. doi: 10.1038/onc.2010.495. Epub 2010 Nov 8. Oncogene. 2011. PMID: 21057528 Free PMC article. - Structure of the leukemia oncogene LMO2: implications for the assembly of a hematopoietic transcription factor complex.
El Omari K, Hoosdally SJ, Tuladhar K, Karia D, Vyas P, Patient R, Porcher C, Mancini EJ. El Omari K, et al. Blood. 2011 Feb 17;117(7):2146-56. doi: 10.1182/blood-2010-07-293357. Epub 2010 Nov 12. Blood. 2011. PMID: 21076045 - The role of LMO2 in development and in T cell leukemia after chromosomal translocation or retroviral insertion.
Nam CH, Rabbitts TH. Nam CH, et al. Mol Ther. 2006 Jan;13(1):15-25. doi: 10.1016/j.ymthe.2005.09.010. Epub 2005 Nov 2. Mol Ther. 2006. PMID: 16260184 Review. - SCL/TAL1 in Hematopoiesis and Cellular Reprogramming.
Hoang T, Lambert JA, Martin R. Hoang T, et al. Curr Top Dev Biol. 2016;118:163-204. doi: 10.1016/bs.ctdb.2016.01.004. Epub 2016 Feb 18. Curr Top Dev Biol. 2016. PMID: 27137657 Review.
Cited by
- In vivo CRISPR/Cas9-mediated screen reveals a critical function of TFDP1 and E2F4 transcription factors in hematopoiesis.
Tran NT, Graf R, Acevedo-Ochoa E, Trombke J, Weber T, Sommermann T, Salomon C, Kühn R, Rajewsky K, Chu VT. Tran NT, et al. Leukemia. 2024 Sep;38(9):2003-2015. doi: 10.1038/s41375-024-02357-w. Epub 2024 Jul 23. Leukemia. 2024. PMID: 39043964 Free PMC article. - Understanding Aberrant Signaling to Elude Therapy Escape Mechanisms in Myeloproliferative Neoplasms.
Bochicchio MT, Di Battista V, Poggio P, Carrà G, Morotti A, Brancaccio M, Lucchesi A. Bochicchio MT, et al. Cancers (Basel). 2022 Feb 15;14(4):972. doi: 10.3390/cancers14040972. Cancers (Basel). 2022. PMID: 35205715 Free PMC article. Review. - Aberrant stem cell and developmental programs in pediatric leukemia.
Ling RE, Cross JW, Roy A. Ling RE, et al. Front Cell Dev Biol. 2024 Mar 27;12:1372899. doi: 10.3389/fcell.2024.1372899. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38601080 Free PMC article. Review. - Transcription factor-mediated reprogramming toward hematopoietic stem cells.
Ebina W, Rossi DJ. Ebina W, et al. EMBO J. 2015 Mar 12;34(6):694-709. doi: 10.15252/embj.201490804. Epub 2015 Feb 20. EMBO J. 2015. PMID: 25712209 Free PMC article. Review. - LIM domain only-2 (LMO2) induces T-cell leukemia by two distinct pathways.
Smith S, Tripathi R, Goodings C, Cleveland S, Mathias E, Hardaway JA, Elliott N, Yi Y, Chen X, Downing J, Mullighan C, Swing DA, Tessarollo L, Li L, Love P, Jenkins NA, Copeland NG, Thompson MA, Du Y, Davé UP. Smith S, et al. PLoS One. 2014 Jan 21;9(1):e85883. doi: 10.1371/journal.pone.0085883. eCollection 2014. PLoS One. 2014. PMID: 24465765 Free PMC article.
References
- Rabbitts T H. Nature (London) 1994;372:143–149. - PubMed
- Royer-Pokora B, Loos U, Ludwig W-D. Oncogene. 1991;6:1887–1893. - PubMed
- Larson R C, Osada H, Larson T A, Lavenir I, Rabbitts T H. Oncogene. 1995;11:853–862. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous